

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50090910 (3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM294045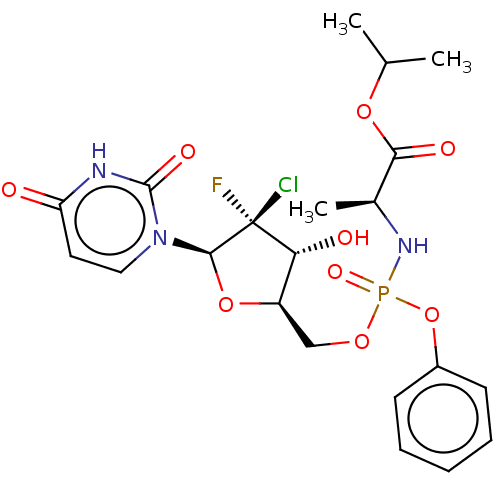 (US10106571, Example 2 | US10106571, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 using ketoconazole as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239940 (CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 using ketoconazole as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50020676 (CHEBI:41846 | CHEMBL566812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50239939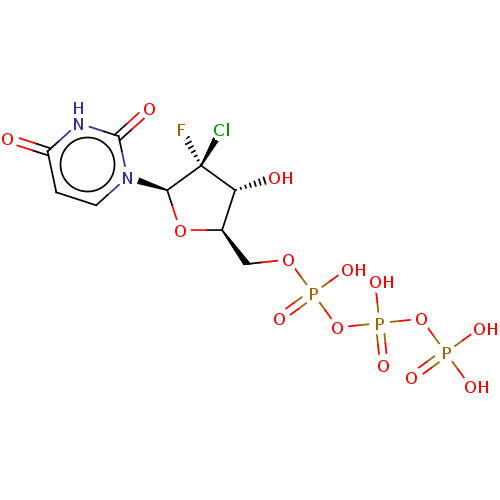 (CHEMBL4088430) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM294045 (US10106571, Example 2 | US10106571, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 using sulfaphenazole as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50239940 (CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 using sulfaphenazole as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50239940 (CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 using alpha-naphthoflavone as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM294045 (US10106571, Example 2 | US10106571, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 using alpha-naphthoflavone as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM294045 (US10106571, Example 2 | US10106571, Example 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 using quinidine as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50239940 (CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 using quinidine as substrate | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50333129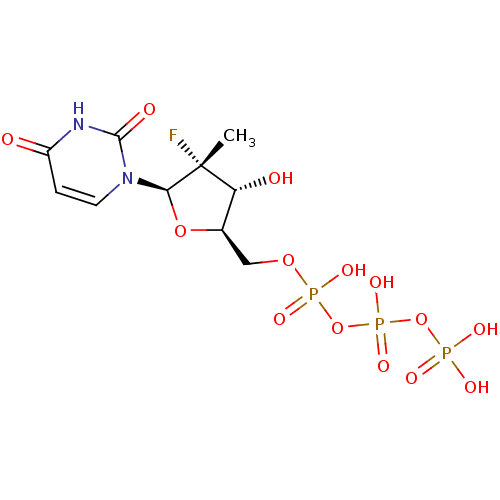 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50239939 (CHEMBL4088430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50333129 (((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis | J Med Chem 60: 5424-5437 (2017) Article DOI: 10.1021/acs.jmedchem.7b00067 BindingDB Entry DOI: 10.7270/Q2BZ6873 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||