

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Bos taurus (bovine)) | BDBM50046899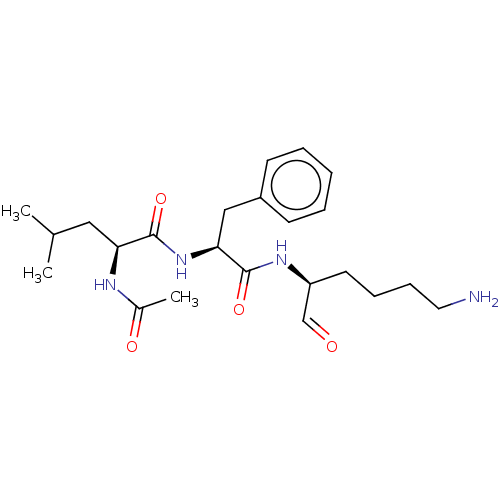 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046896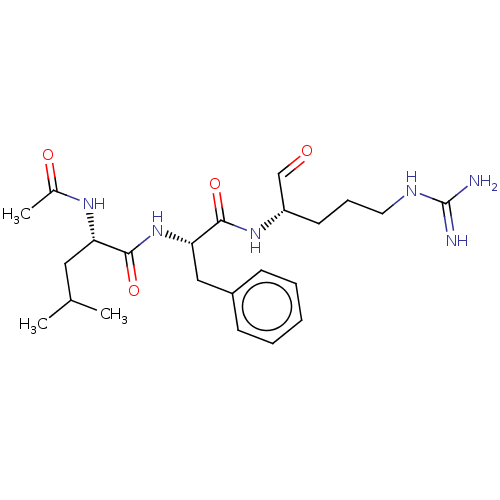 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046896 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046896 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046896 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046887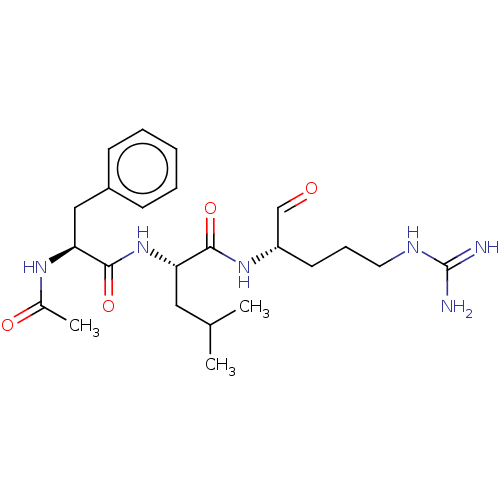 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046887 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50046887 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of trypsin with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046887 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046887 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046888 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of trypsin with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046885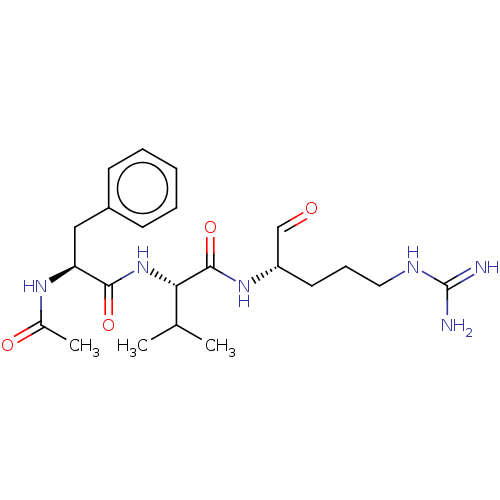 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-for...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046885 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-for...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50046885 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-for...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of trypsin with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046885 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-for...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046885 (2-(2-Acetylamino-3-phenyl-propionylamino)-N-(1-for...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046890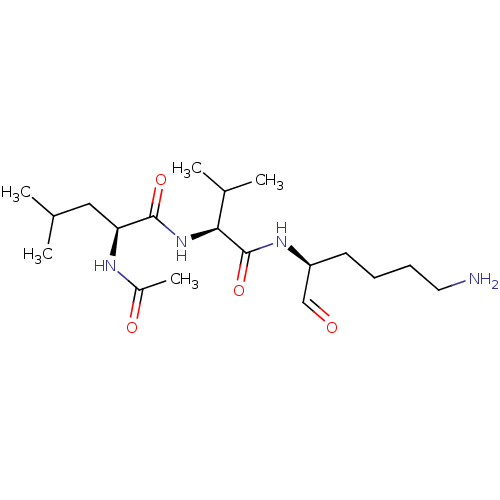 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046890 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046890 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50046890 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of trypsin with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046888 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50046891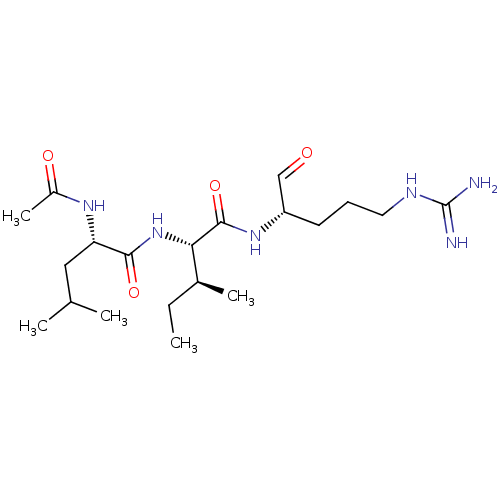 (2-(2-Acetylamino-4-methyl-pentanoylamino)-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of trypsin with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046891 (2-(2-Acetylamino-4-methyl-pentanoylamino)-3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046891 (2-(2-Acetylamino-4-methyl-pentanoylamino)-3-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046891 (2-(2-Acetylamino-4-methyl-pentanoylamino)-3-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046898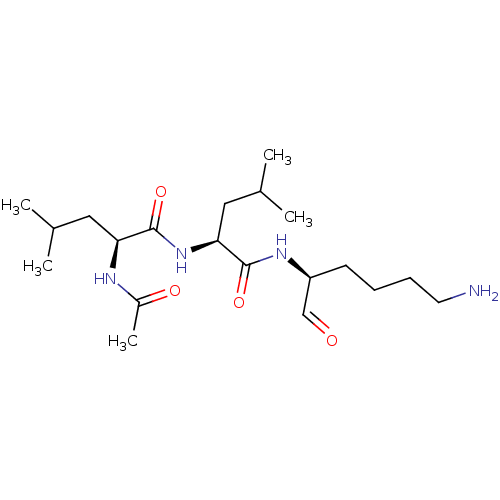 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046900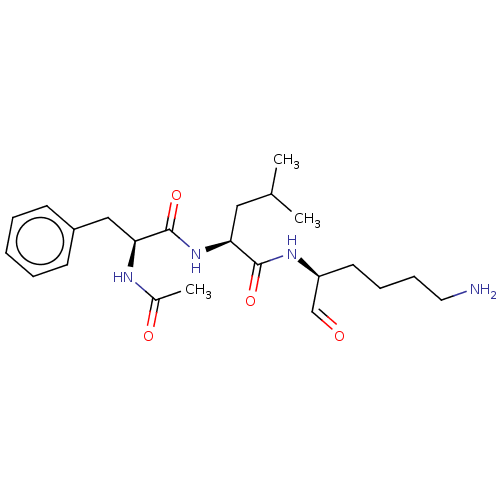 (2-(2-Acetylamino-3-phenyl-propionylamino)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046891 (2-(2-Acetylamino-4-methyl-pentanoylamino)-3-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046898 (2-Acetylamino-4-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046895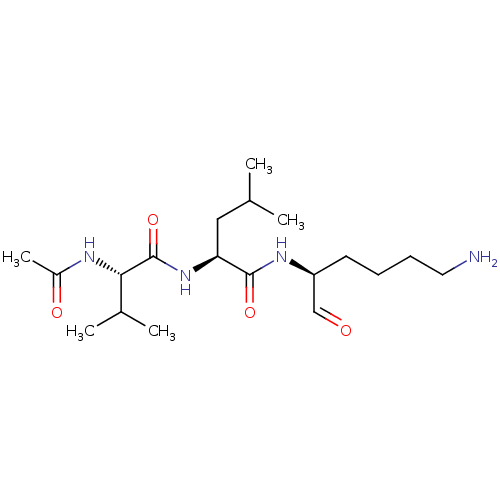 (2-Acetylamino-3-methyl-pentanoic acid [1-(5-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046897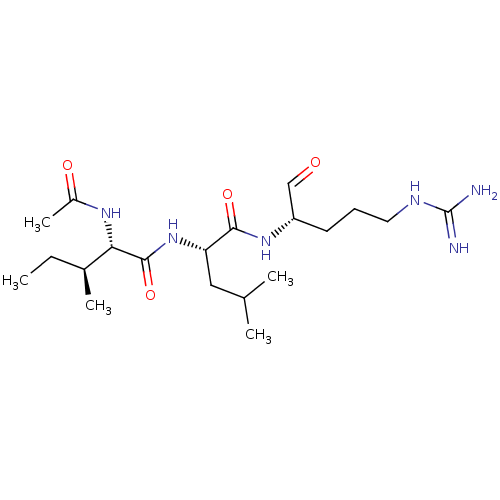 (2-Acetylamino-3-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046897 (2-Acetylamino-3-methyl-pentanoic acid [1-(1-formyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046897 (2-Acetylamino-3-methyl-pentanoic acid [1-(1-formyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046894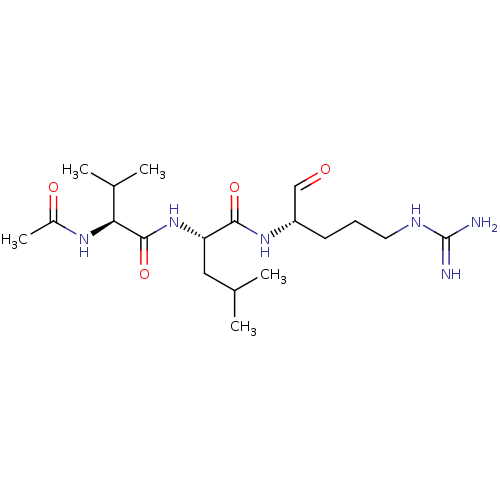 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50046894 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of trypsin with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046894 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046894 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046894 (2-(2-Acetylamino-3-methyl-butyrylamino)-4-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50046892 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of thrombin with benzoyl-Phe-Val-arginine- p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046892 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046892 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046892 (2-Acetylamino-4-methyl-pentanoic acid [1-(1-formyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM50046893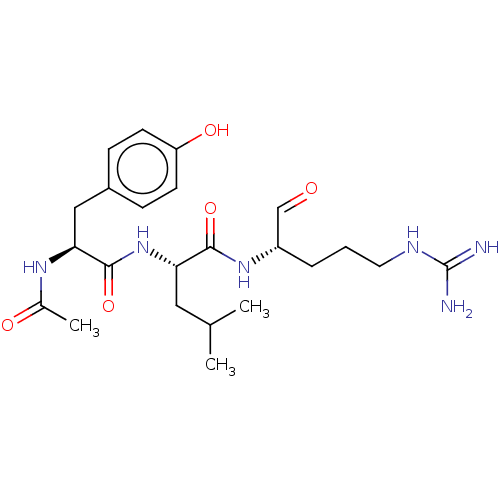 (2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of cathepsin B with Cbz-L-lysine-p-nitrophenyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50046893 (2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propionylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of kallikrein with benzoyl-L-arginine ethyl ester as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50046893 (2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Inhibition of plasmin with Val-L-Leu-L-lysine-p-nitroanilide as substrate | J Med Chem 36: 1084-9 (1993) BindingDB Entry DOI: 10.7270/Q24F1RCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |