

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate measured after 8 mins in presence of BuChE inhibitor ethopropazine by Ellma... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50245716 (CHEMBL4084610) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433322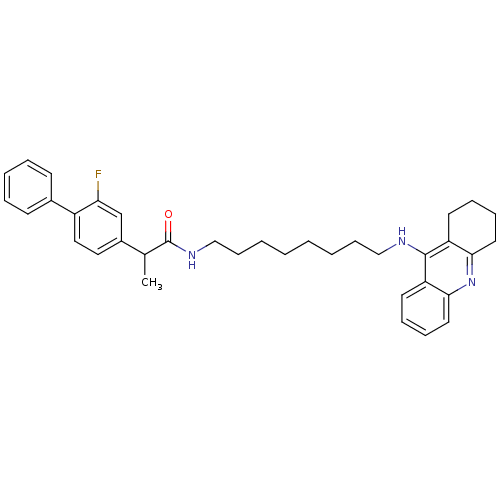 (CHEMBL2376473) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition after 2 mins by DTN... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50235800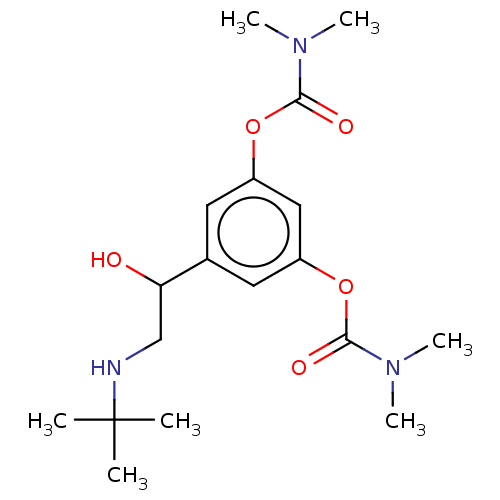 (Bambuterol | CHEBI:553827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50245728 (CHEMBL4063881) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using s-butyrylthiocholine iodide as substrate pretreated for 6 mins followed by substrate addition measured at 60 s... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50245718 (CHEMBL4095755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human plasma AChE using acetyl-(beta-methyl)thiocholine as substrate pretreated for 30 mins followed by substrate addition after 25 min... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8970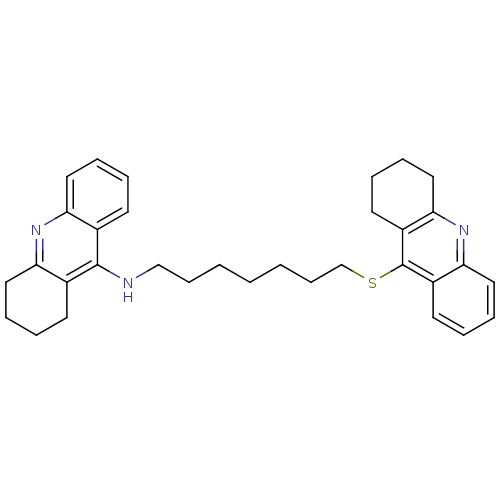 (CHEMBL51197 | N-[7-(1,2,3,4-tetrahydroacridin-9-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50433322 (CHEMBL2376473) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition after 2 mins by DTNB... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245717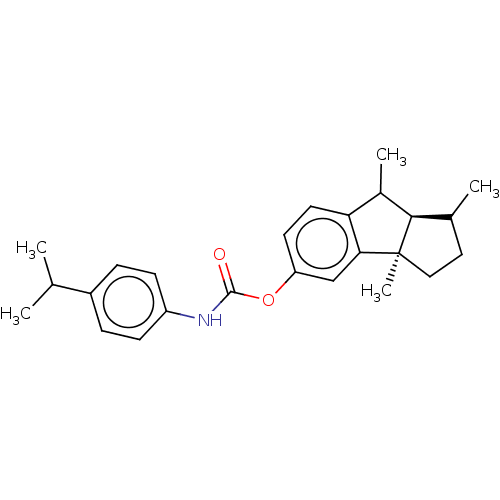 (CHEMBL4068410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human RBC BuChE using s-butyrylthiocholine as substrate pretreated for 30 mins followed by substrate addition after 25 mins by spectrop... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245727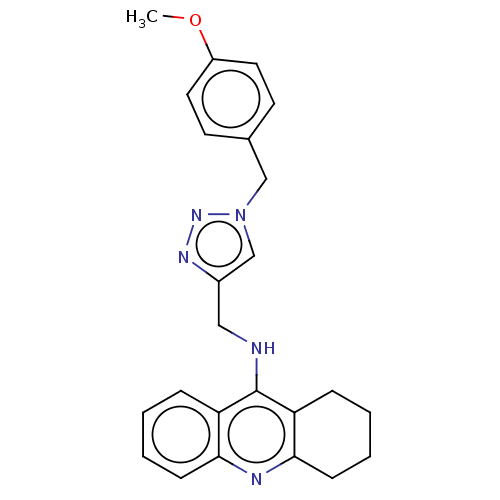 (CHEMBL4063321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human BuChE | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199199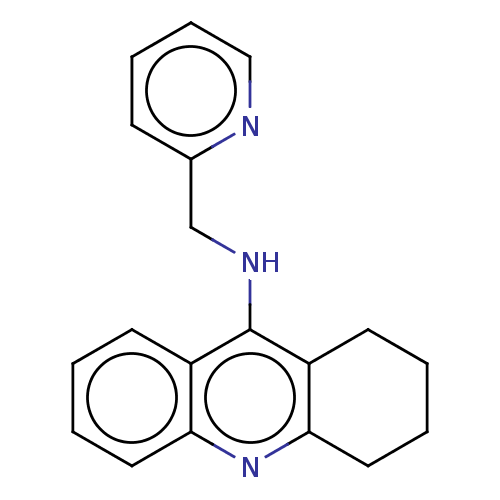 (N-(Pyridin-2-ylmethyl)-1,2,3,4-tetrahydroacridin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured at 1 min i... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50245728 (CHEMBL4063881) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 6 mins followed by substrate measured at 60 secs interval ... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245715 (CHEMBL4072478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8970 (CHEMBL51197 | N-[7-(1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of fetal bovine serum AChE using acetylthiocholine iodide as substrate | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10520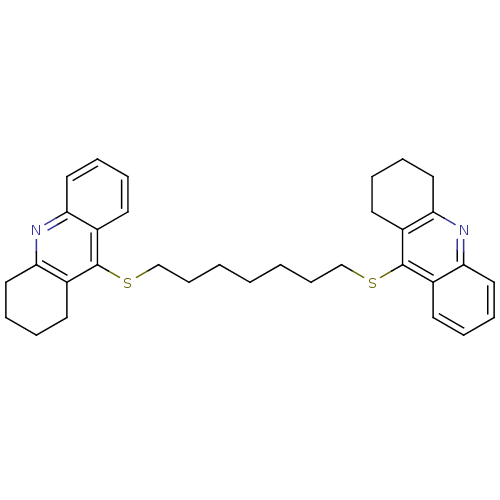 (9-{[7-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)hept...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50245736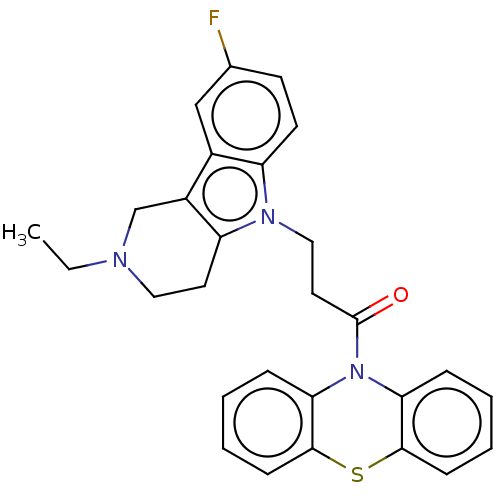 (CHEMBL4099400) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition by DTNB reagent b... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50245717 (CHEMBL4068410) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human plasma AChE using acetyl-(beta-methyl)thiocholine as substrate pretreated for 30 mins followed by substrate addition after 25 min... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50245716 (CHEMBL4084610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of fetal bovine serum AChE using acetylthiocholine iodide as substrate | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245718 (CHEMBL4095755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human RBC BuChE using s-butyrylthiocholine as substrate pretreated for 30 mins followed by substrate addition after 25 mins by spectrop... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50245727 (CHEMBL4063321) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50245715 (CHEMBL4072478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine chloride as substrate after 5 mins by spectrophotometric metho... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245709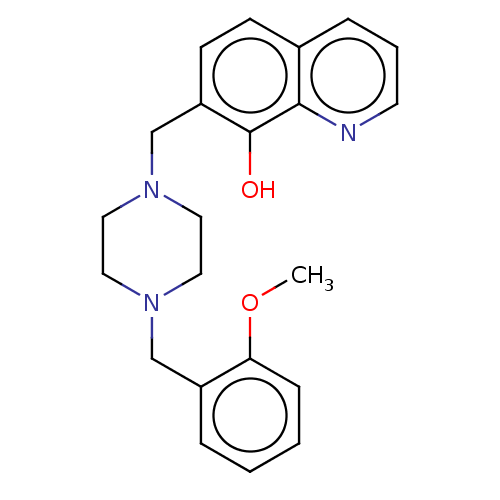 (CHEMBL4082964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 20 mins followed by substrate addition by DTNB reagent ba... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM199199 (N-(Pyridin-2-ylmethyl)-1,2,3,4-tetrahydroacridin-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured at 1 ... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245730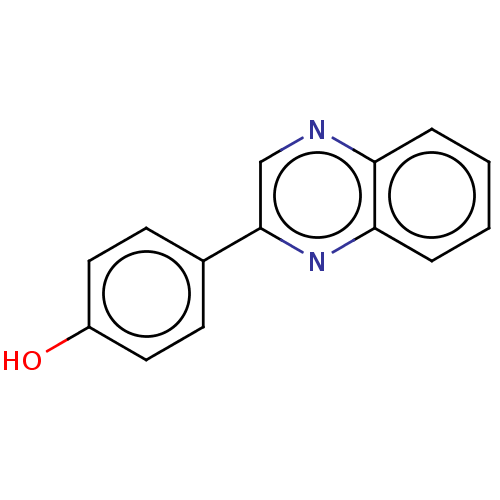 (CHEMBL1730860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10520 (9-{[7-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of fetal bovine serum AChE using acetylthiocholine iodide as substrate | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50245708 (CHEMBL4067633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate measured at 12 secs interval for 10 mins by Ellman's method | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50235800 (Bambuterol | CHEBI:553827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245735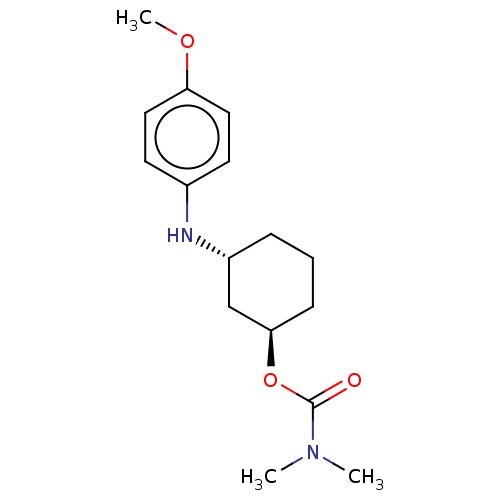 (CHEMBL4079247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine iodine as substrate pretreated for 10 mins followed by substrate addition measured for 5 mi... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50245729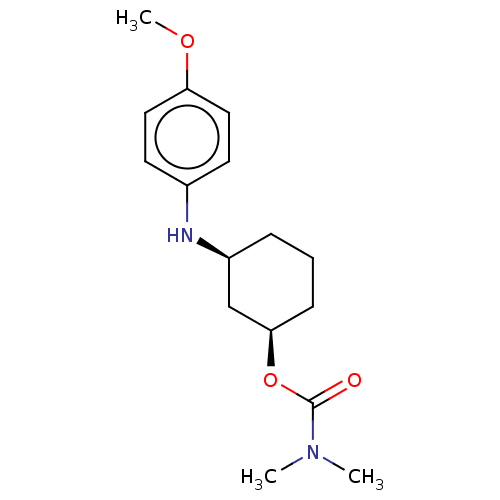 (CHEMBL4080162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine iodine as substrate pretreated for 10 mins followed by substrate addition measured for 5 mi... | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate measured at 12 secs interval for 10 mins by Ellman's method | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50245708 (CHEMBL4067633) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured at 12 secs interval for 10 mins by Ellman's method | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured at 12 secs interval for 10 mins by Ellman's method | Eur J Med Chem 132: 294-309 (2017) Article DOI: 10.1016/j.ejmech.2017.03.062 BindingDB Entry DOI: 10.7270/Q2HX1G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||