

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50247634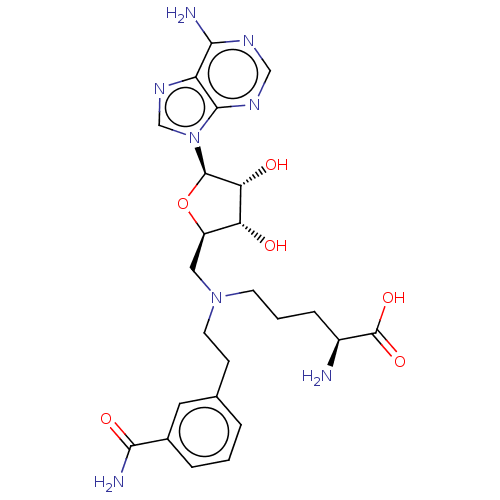 (CHEMBL4067973) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of DOT1L (unknown origin) after 1 hr by filter-based assay | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50247634 (CHEMBL4067973) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human NNMT (1 to 264 residues) expressed in Escherichia coli BL21 (DE3) using nicotinamide/SAM as substrate/co-factor measured for 20 m... | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 7 (Homo sapiens (Human)) | BDBM50247634 (CHEMBL4067973) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of PRMT7 (unknown origin) using [3H]-SAM as co-factor by scintillation proximity assay | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA 5'-monophosphate methyltransferase (Homo sapiens) | BDBM50247634 (CHEMBL4067973) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of BCDIN3D (unknown origin) using [3H]-SAM as co-factor by scintillation proximity assay | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50247634 (CHEMBL4067973) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using [3H]-SAM as co-factor by scintillation proximity assay | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50247635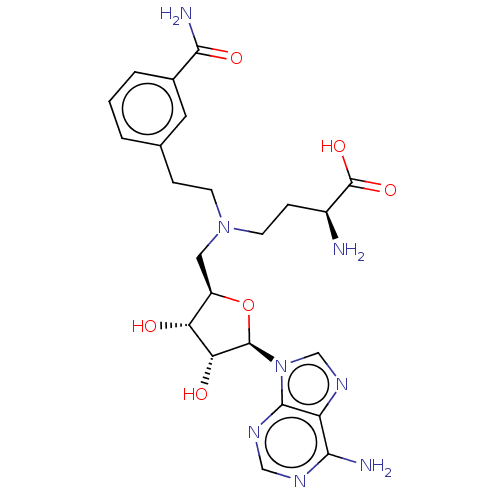 (CHEMBL4100002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human NNMT (1 to 264 residues) expressed in Escherichia coli BL21 (DE3) using nicotinamide/SAM as substrate/co-factor measured for 20 m... | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50247634 (CHEMBL4067973) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Binding affinity to human NNMT (1 to 264 residues) expressed in Escherichia coli BL21 (DE3) by isothermal titration calorimetric method | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50247635 (CHEMBL4100002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.28E+4 | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Binding affinity to human NNMT (1 to 264 residues) expressed in Escherichia coli BL21 (DE3) by isothermal titration calorimetric method | J Med Chem 61: 1541-1551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01422 BindingDB Entry DOI: 10.7270/Q2FT8PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||