 Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50036458
Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50036458 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435
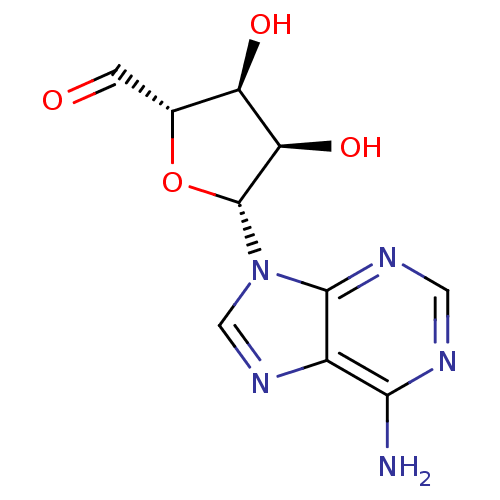
(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369159
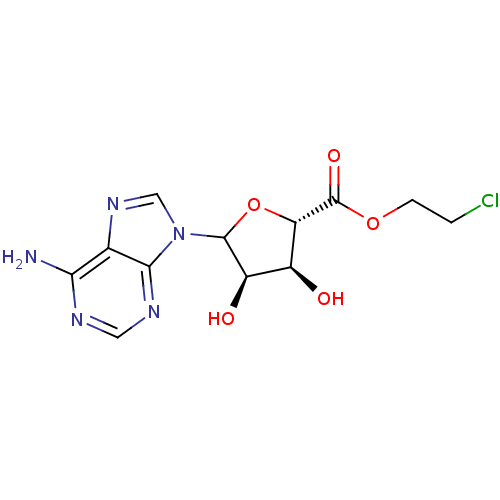
(CHEMBL610148)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)OCCCl |r| Show InChI InChI=1S/C12H14ClN5O5/c13-1-2-22-12(21)8-6(19)7(20)11(23-8)18-4-17-5-9(14)15-3-16-10(5)18/h3-4,6-8,11,19-20H,1-2H2,(H2,14,15,16)/t6-,7+,8-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369158
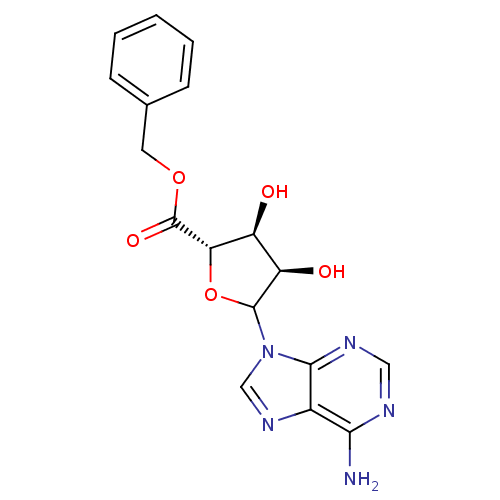
(CHEMBL612224)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C17H17N5O5/c18-14-10-15(20-7-19-14)22(8-21-10)16-12(24)11(23)13(27-16)17(25)26-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,16,23-24H,6H2,(H2,18,19,20)/t11-,12+,13-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50331791
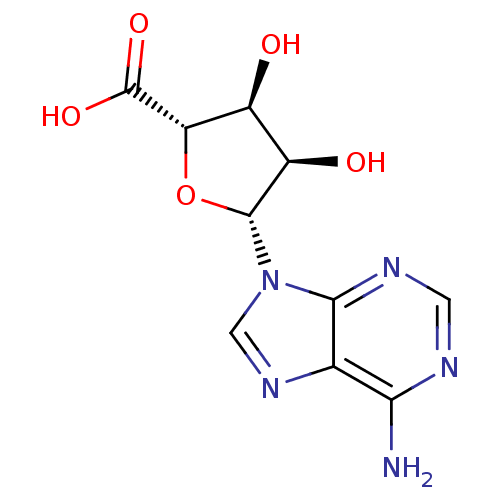
((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(O)=O |r| Show InChI InChI=1S/C10H11N5O5/c11-7-3-8(13-1-12-7)15(2-14-3)9-5(17)4(16)6(20-9)10(18)19/h1-2,4-6,9,16-17H,(H,18,19)(H2,11,12,13)/t4-,5+,6-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369156
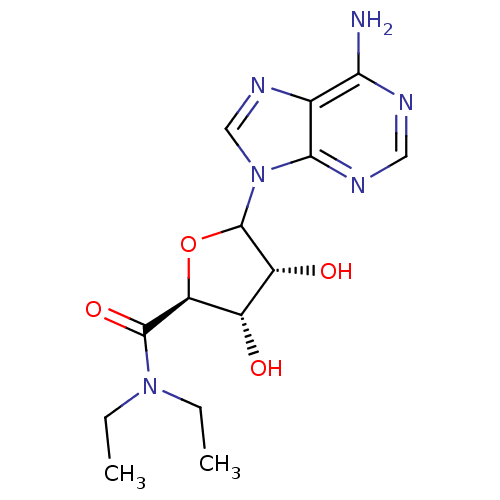
(CHEMBL608072)Show SMILES CCN(CC)C(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N6O4/c1-3-19(4-2)13(23)10-8(21)9(22)14(24-10)20-6-18-7-11(15)16-5-17-12(7)20/h5-6,8-10,14,21-22H,3-4H2,1-2H3,(H2,15,16,17)/t8-,9+,10-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369161
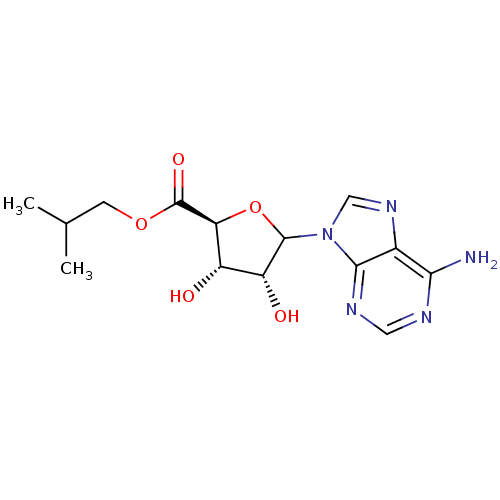
(CHEMBL610383)Show SMILES CC(C)COC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N5O5/c1-6(2)3-23-14(22)10-8(20)9(21)13(24-10)19-5-18-7-11(15)16-4-17-12(7)19/h4-6,8-10,13,20-21H,3H2,1-2H3,(H2,15,16,17)/t8-,9+,10-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369162
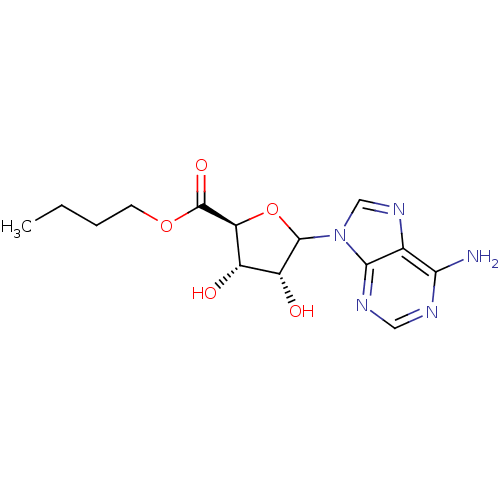
(CHEMBL610384)Show SMILES CCCCOC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N5O5/c1-2-3-4-23-14(22)10-8(20)9(21)13(24-10)19-6-18-7-11(15)16-5-17-12(7)19/h5-6,8-10,13,20-21H,2-4H2,1H3,(H2,15,16,17)/t8-,9+,10-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369157
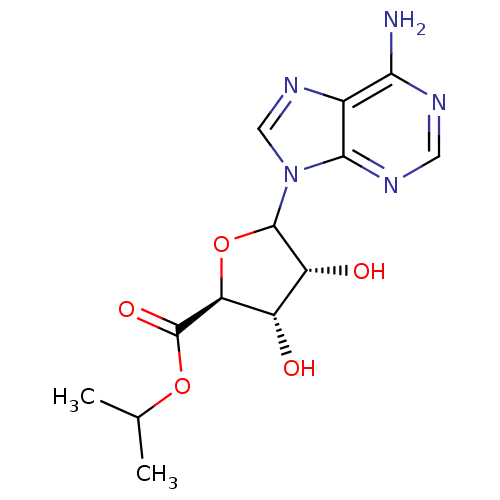
(CHEMBL608915)Show SMILES CC(C)OC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C13H17N5O5/c1-5(2)22-13(21)9-7(19)8(20)12(23-9)18-4-17-6-10(14)15-3-16-11(6)18/h3-5,7-9,12,19-20H,1-2H3,(H2,14,15,16)/t7-,8+,9-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369160
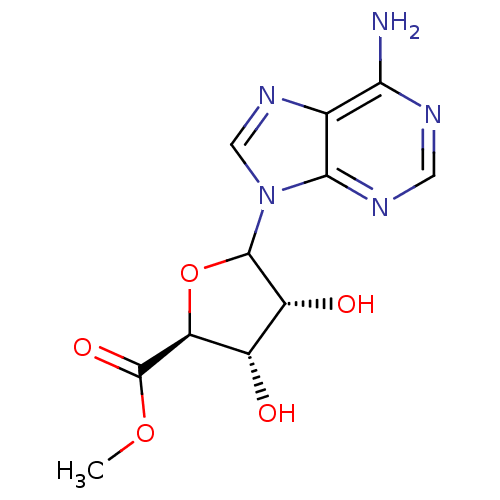
(CHEMBL608025)Show SMILES COC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13N5O5/c1-20-11(19)7-5(17)6(18)10(21-7)16-3-15-4-8(12)13-2-14-9(4)16/h2-3,5-7,10,17-18H,1H3,(H2,12,13,14)/t5-,6+,7-,10?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407975
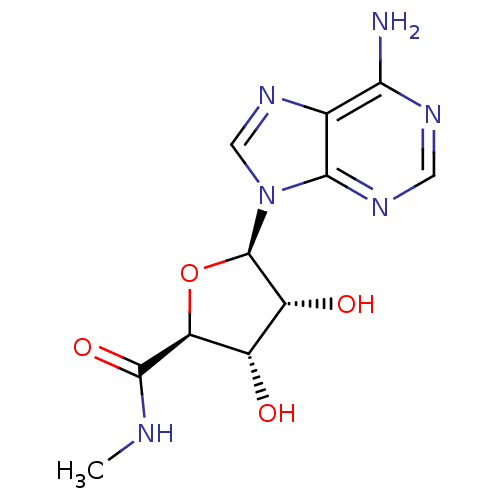
(CHEMBL519809)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14N6O4/c1-13-10(20)7-5(18)6(19)11(21-7)17-3-16-4-8(12)14-2-15-9(4)17/h2-3,5-7,11,18-19H,1H3,(H,13,20)(H2,12,14,15)/t5-,6+,7-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50367376
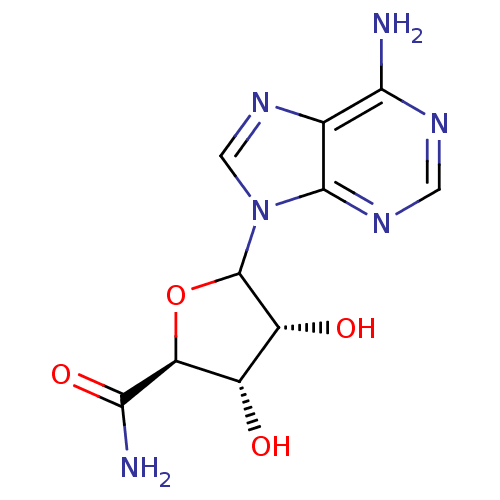
(CHEMBL605866)Show SMILES NC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C10H12N6O4/c11-7-3-9(14-1-13-7)16(2-15-3)10-5(18)4(17)6(20-10)8(12)19/h1-2,4-6,10,17-18H,(H2,12,19)(H2,11,13,14)/t4-,5+,6-,10?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369155
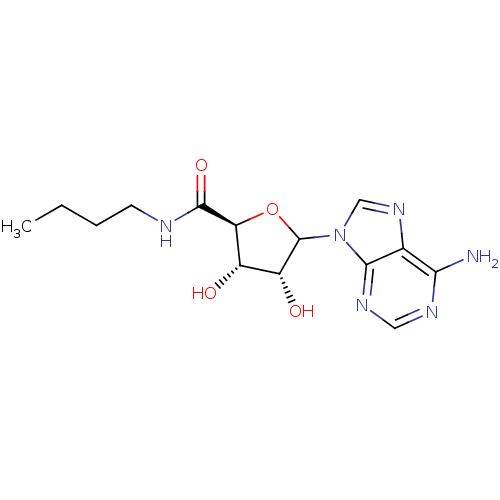
(CHEMBL609186)Show SMILES CCCCNC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N6O4/c1-2-3-4-16-13(23)10-8(21)9(22)14(24-10)20-6-19-7-11(15)17-5-18-12(7)20/h5-6,8-10,14,21-22H,2-4H2,1H3,(H,16,23)(H2,15,17,18)/t8-,9+,10-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM85777
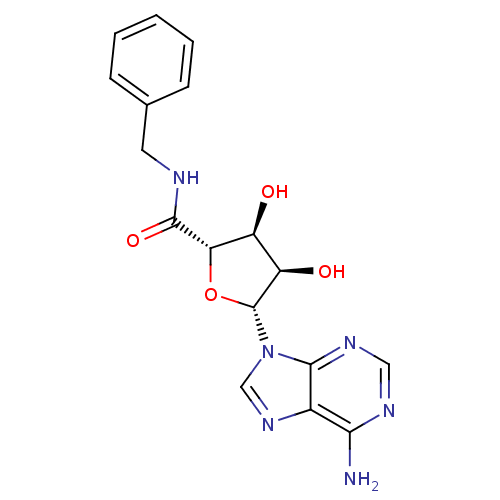
(B-NECA)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C17H18N6O4/c18-14-10-15(21-7-20-14)23(8-22-10)17-12(25)11(24)13(27-17)16(26)19-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,17,24-25H,6H2,(H,19,26)(H2,18,20,21)/t11-,12+,13-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369163
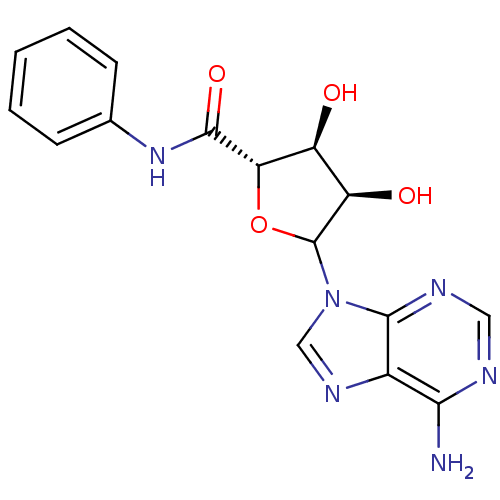
(CHEMBL610101)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C16H16N6O4/c17-13-9-14(19-6-18-13)22(7-20-9)16-11(24)10(23)12(26-16)15(25)21-8-4-2-1-3-5-8/h1-7,10-12,16,23-24H,(H,21,25)(H2,17,18,19)/t10-,11+,12-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data