

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (MOUSE) | BDBM50058154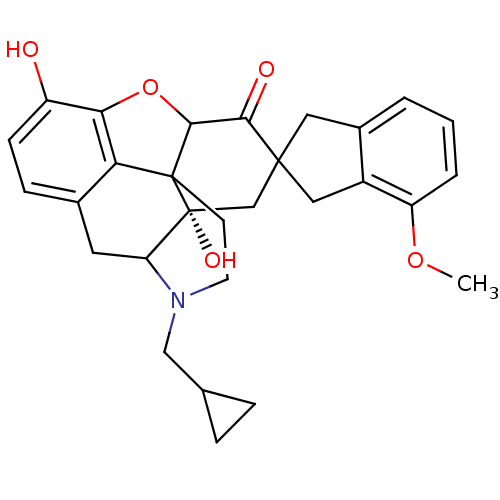 (7-(4'-Methoxy-2'-spiroindanyl)naltrexone | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50058152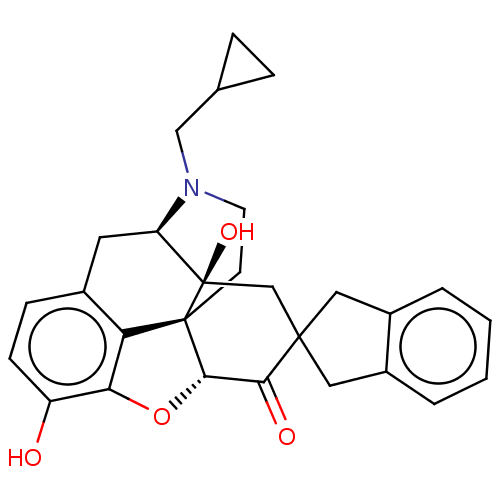 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058154 (7-(4'-Methoxy-2'-spiroindanyl)naltrexone | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50058152 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50297170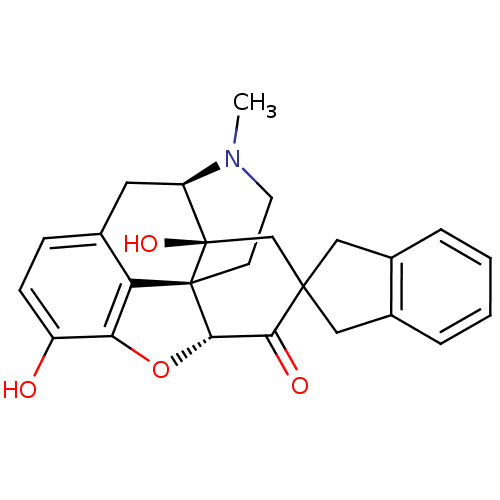 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50058149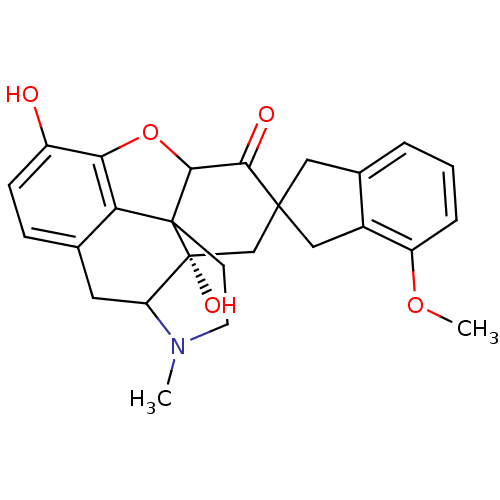 (7-(4'-Methoxy-2'-spiroindanyl)oxymorphone | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor delta 1 using [3H]- NT1 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058150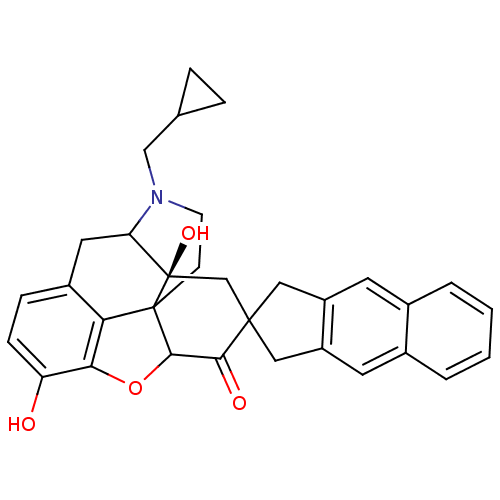 (7-(5',6'-Benzo-2'-spiroindanyl)naltrexone | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058149 (7-(4'-Methoxy-2'-spiroindanyl)oxymorphone | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50297170 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50058153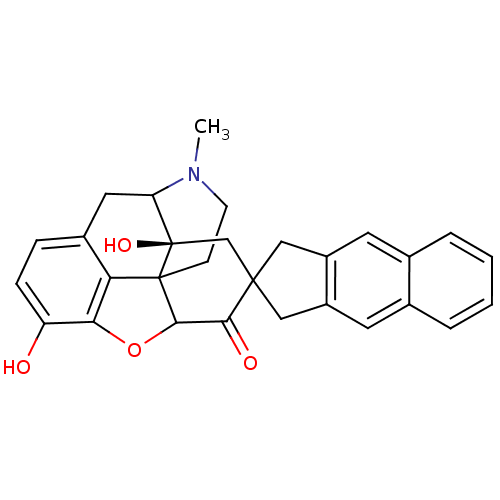 (7-(5',6'-Benzo-2'-spiroindanyl)oxymorphone | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against [3H]- NT1 (Opioid receptor delta 1) opioid receptor | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50058153 (7-(5',6'-Benzo-2'-spiroindanyl)oxymorphone | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor mu 1 using [3H]- DAMGO as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50058150 (7-(5',6'-Benzo-2'-spiroindanyl)naltrexone | CHEMBL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor kappa 1 using [3H]U-69,594 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50297170 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor kappa 1 as the [3H]- U-69,594 radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50058154 (7-(4'-Methoxy-2'-spiroindanyl)naltrexone | CHEMBL2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor kappa 1 using [3H]U-69,594 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50058153 (7-(5',6'-Benzo-2'-spiroindanyl)oxymorphone | CHEMB...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor kappa 1 using [3H]U-69,594 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50058152 (7-(2''-spiroindanyl)naltrexone | CHEMBL610293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor kappa 1 as the [3H]- U-69,594 radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50058149 (7-(4'-Methoxy-2'-spiroindanyl)oxymorphone | CHEMBL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description The compound tested for agonistic activity against Opioid receptor kappa 1 using [3H]U-69,594 as the radioligand. | J Med Chem 40: 1720-5 (1997) Article DOI: 10.1021/jm9700880 BindingDB Entry DOI: 10.7270/Q2FB53M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||