

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065807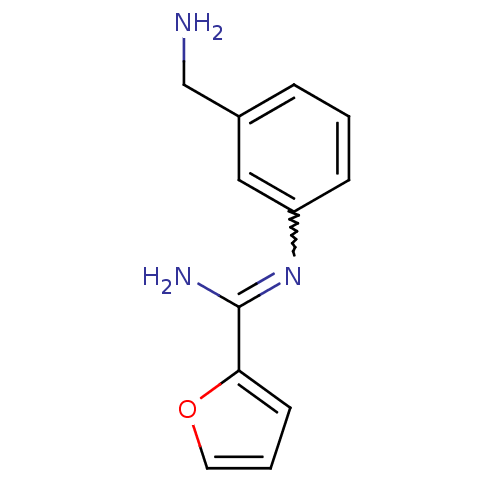 (CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065843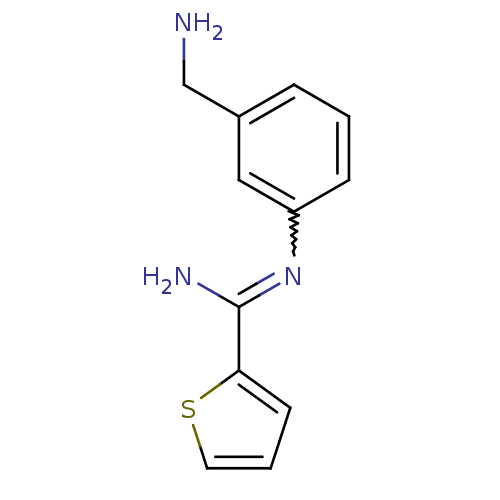 (CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065823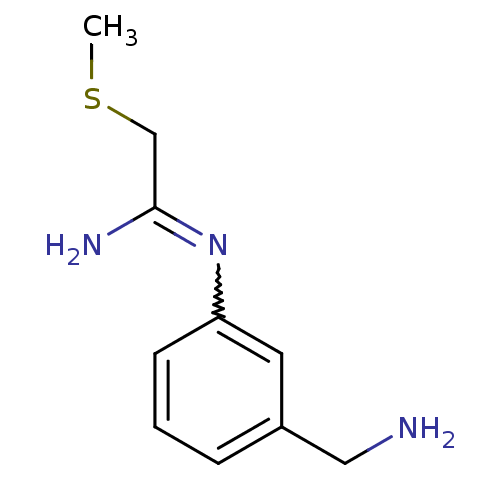 (CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065813 (CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50225106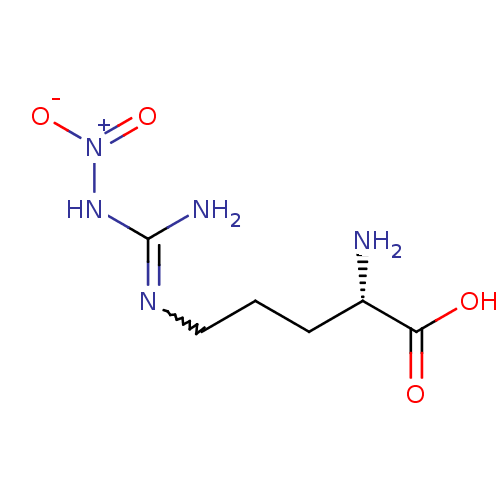 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065823 (CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065833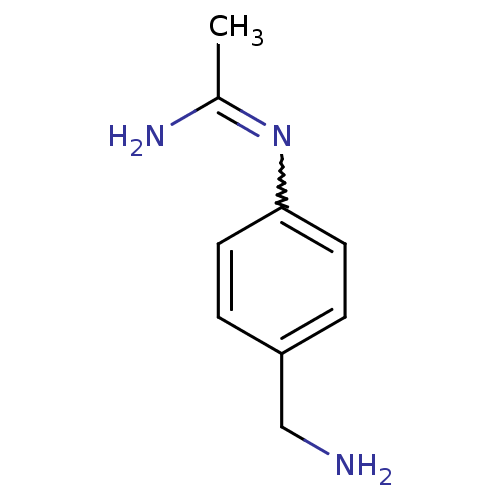 (CHEMBL542432 | N-(4-Aminomethyl-phenyl)-acetamidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065842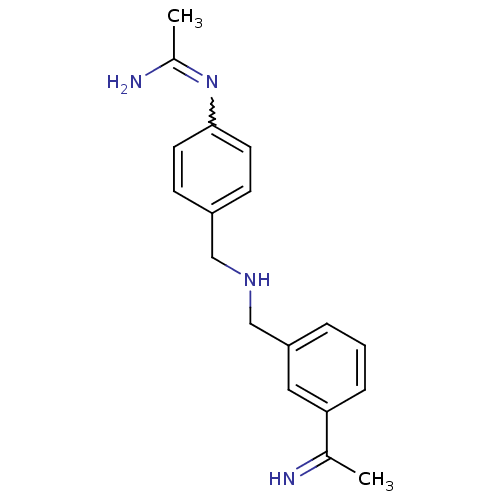 (CHEMBL540048 | N-(4-{[3-(1-Imino-ethyl)-benzylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065848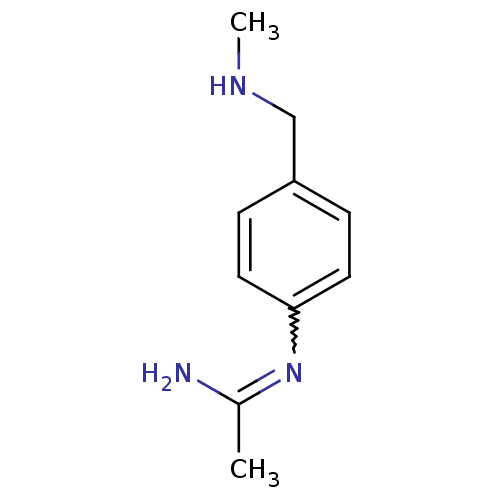 (CHEMBL539793 | N-(4-Methylaminomethyl-phenyl)-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065843 (CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065805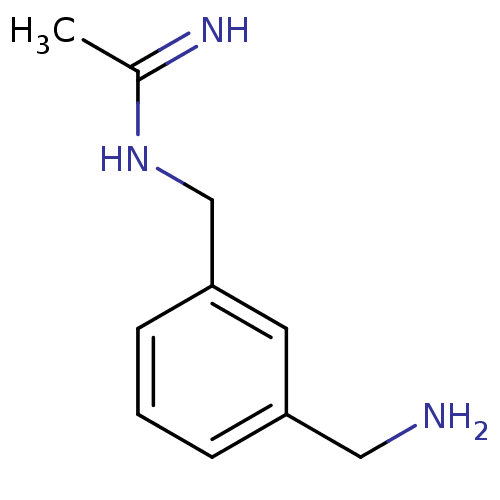 (CHEMBL544788 | N-(3-Aminomethyl-benzyl)-acetamidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065807 (CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065818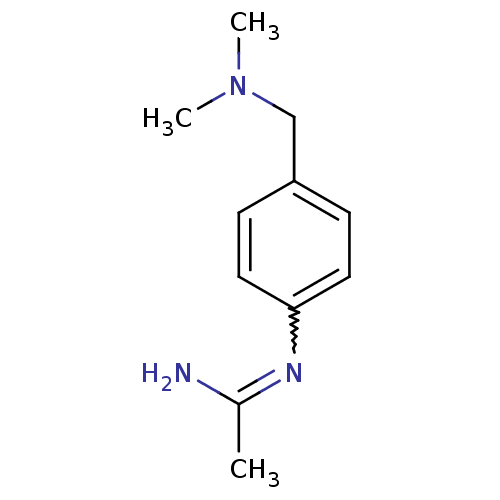 (CHEMBL554201 | N-(4-Dimethylaminomethyl-phenyl)-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065817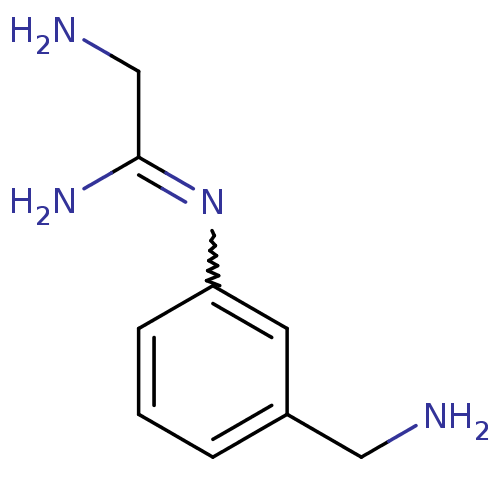 (2-Amino-N-(3-aminomethyl-phenyl)-acetamidine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065847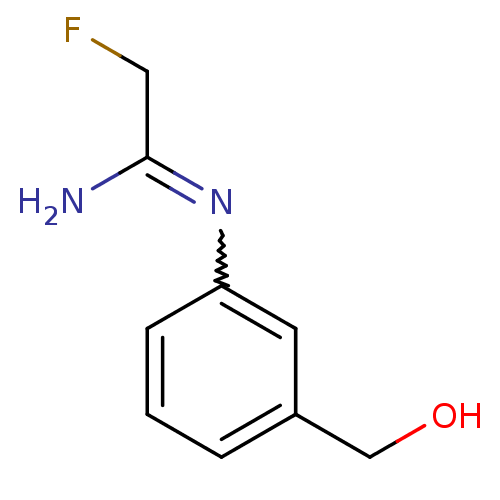 (2-Fluoro-N-(3-hydroxymethyl-phenyl)-acetamidine | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065823 (CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065812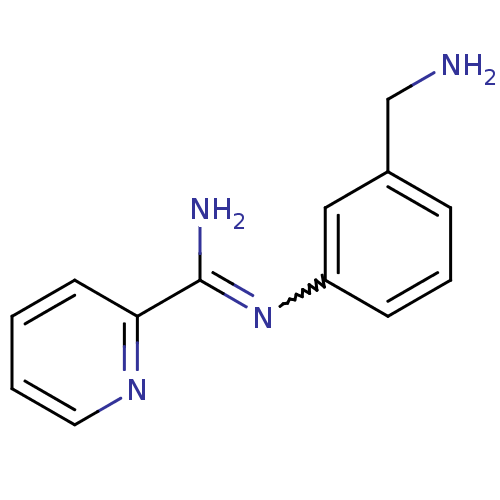 (CHEMBL544793 | N-(3-Aminomethyl-phenyl)-pyridine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065807 (CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065809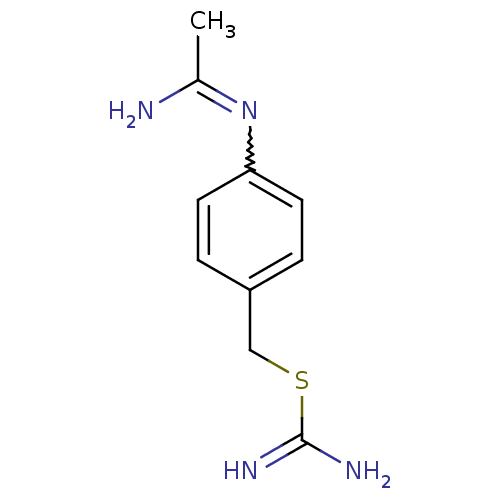 (CHEMBL544317 | N-(4-Carbamimidoylsulfanylmethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065841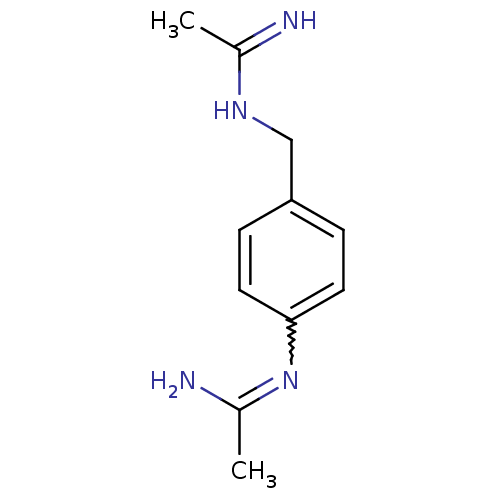 (CHEMBL545025 | N-[4-(Acetimidoylamino-methyl)-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065843 (CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065813 (CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065815 (4-Acetimidoylamino-benzamidine | CHEMBL543380) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065803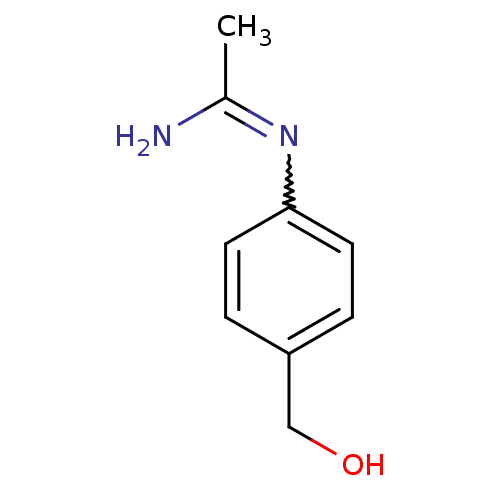 (CHEMBL542431 | N-(4-Hydroxymethyl-phenyl)-acetamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065838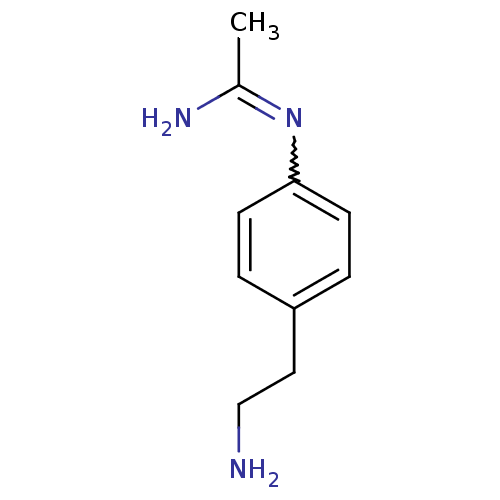 (CHEMBL538767 | N-[4-(2-Amino-ethyl)-phenyl]-acetam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065826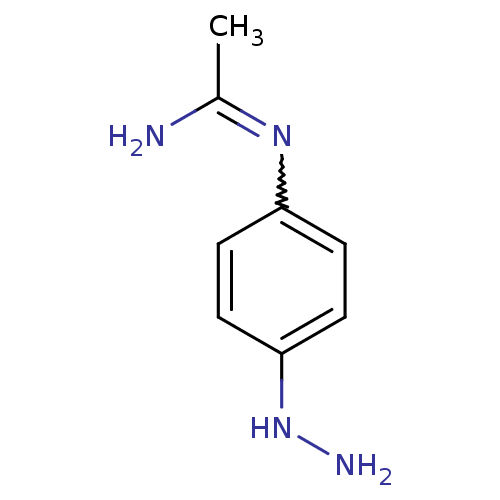 (CHEMBL543142 | N-(4-Hydrazino-phenyl)-acetamidine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065813 (CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065824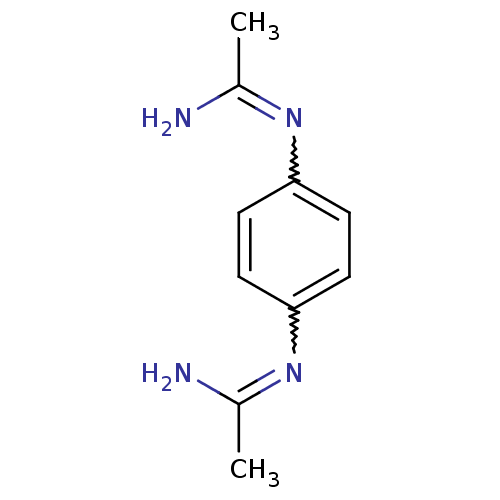 (CHEMBL544090 | N-(4-Acetimidoylamino-phenyl)-aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065828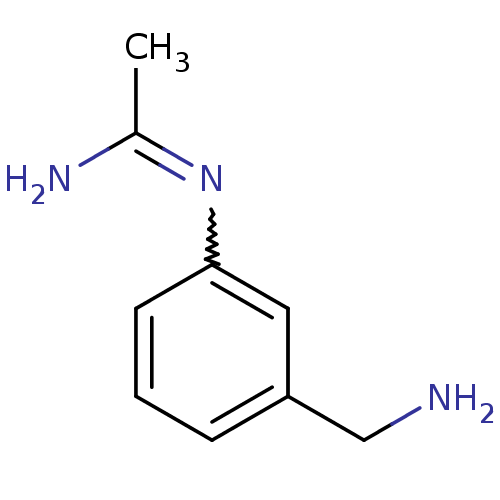 (CHEMBL542678 | N-(3-Aminomethyl-phenyl)-acetamidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065842 (CHEMBL540048 | N-(4-{[3-(1-Imino-ethyl)-benzylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065848 (CHEMBL539793 | N-(4-Methylaminomethyl-phenyl)-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065805 (CHEMBL544788 | N-(3-Aminomethyl-benzyl)-acetamidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065841 (CHEMBL545025 | N-[4-(Acetimidoylamino-methyl)-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065833 (CHEMBL542432 | N-(4-Aminomethyl-phenyl)-acetamidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065827 (CHEMBL538021 | N-(4-Hydroxyaminomethyl-phenyl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065848 (CHEMBL539793 | N-(4-Methylaminomethyl-phenyl)-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065828 (CHEMBL542678 | N-(3-Aminomethyl-phenyl)-acetamidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065829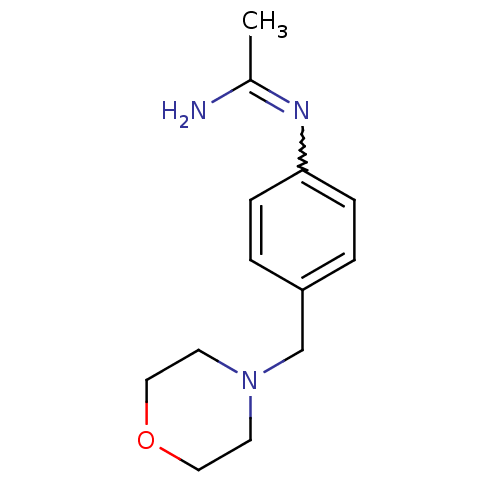 (CHEMBL542676 | N-(4-Morpholin-4-ylmethyl-phenyl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065842 (CHEMBL540048 | N-(4-{[3-(1-Imino-ethyl)-benzylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065817 (2-Amino-N-(3-aminomethyl-phenyl)-acetamidine | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50065847 (2-Fluoro-N-(3-hydroxymethyl-phenyl)-acetamidine | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065809 (CHEMBL544317 | N-(4-Carbamimidoylsulfanylmethyl-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065816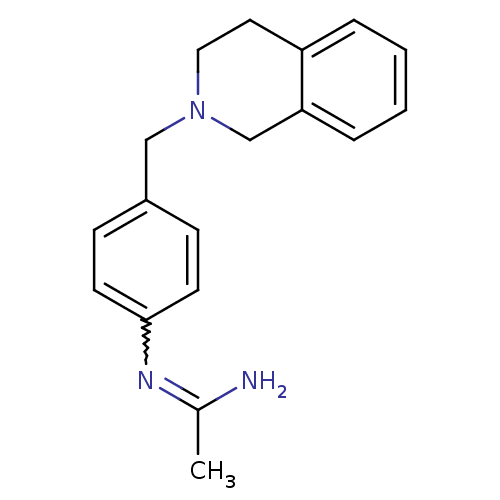 (CHEMBL542910 | N-[4-(3,4-Dihydro-1H-isoquinolin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50065826 (CHEMBL543142 | N-(4-Hydrazino-phenyl)-acetamidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50065830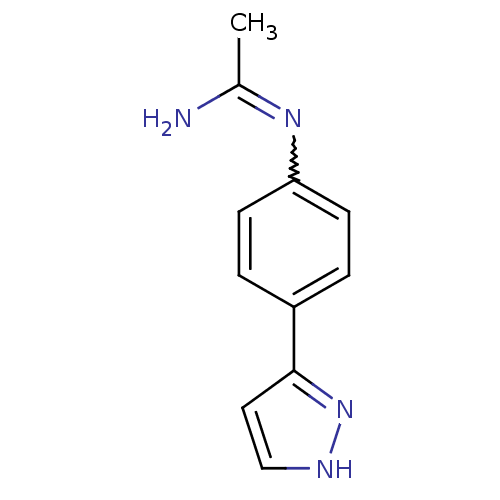 (CHEMBL543381 | N-[4-(2H-Pyrazol-3-yl)-phenyl]-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. | J Med Chem 41: 2858-71 (1998) Article DOI: 10.1021/jm980072p BindingDB Entry DOI: 10.7270/Q2862H4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |