

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252993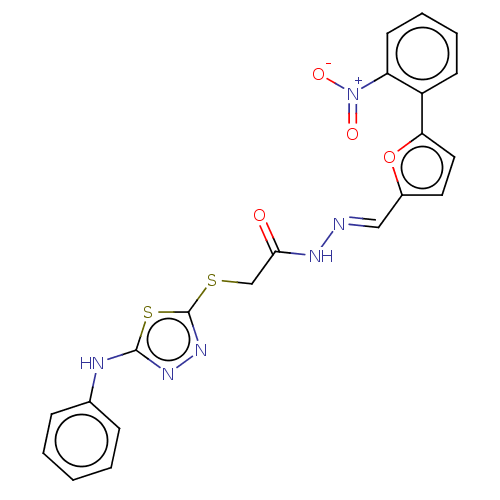 (CHEMBL4073678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252992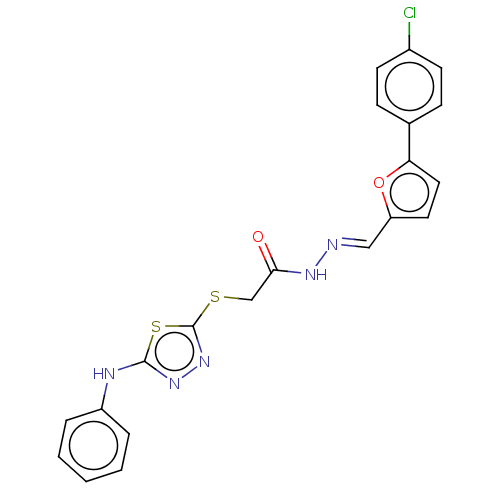 (CHEMBL4100496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252992 (CHEMBL4100496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252996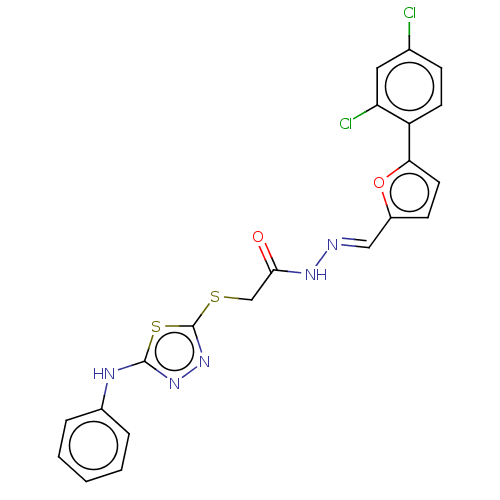 (CHEMBL4097972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252994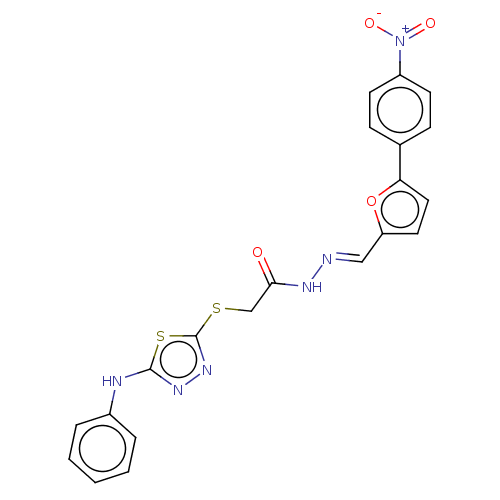 (CHEMBL4082551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252998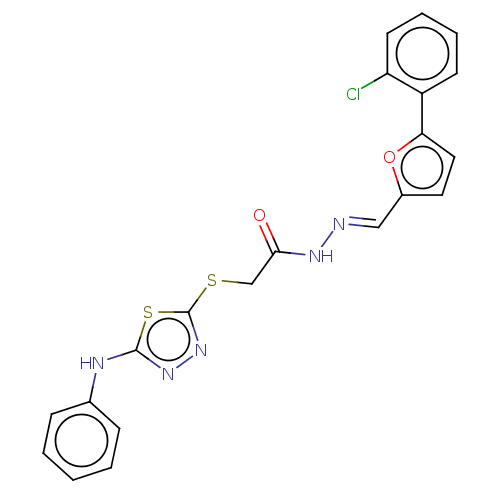 (CHEMBL4105008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252996 (CHEMBL4097972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50253004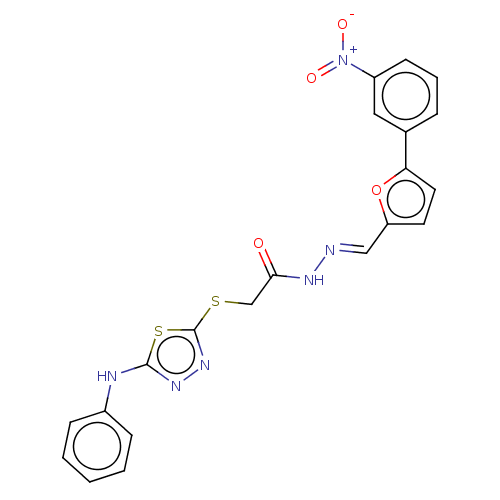 (CHEMBL4072122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253005 (CHEMBL4076887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252993 (CHEMBL4073678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253004 (CHEMBL4072122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Non-competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Linew... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252997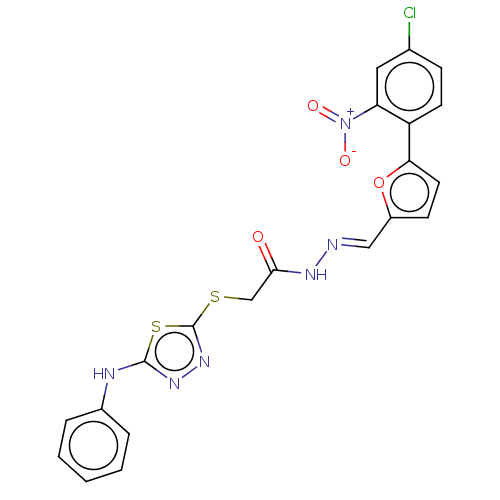 (CHEMBL4098116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252998 (CHEMBL4105008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Competitive inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by Lineweave... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252992 (CHEMBL4100496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252998 (CHEMBL4105008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252992 (CHEMBL4100496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252993 (CHEMBL4073678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252997 (CHEMBL4098116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252994 (CHEMBL4082551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252996 (CHEMBL4097972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252993 (CHEMBL4073678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252996 (CHEMBL4097972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253005 (CHEMBL4076887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50253004 (CHEMBL4072122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253004 (CHEMBL4072122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252998 (CHEMBL4105008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||