 Found 295 hits Enz. Inhib. hit(s) with all data for entry = 50036972
Found 295 hits Enz. Inhib. hit(s) with all data for entry = 50036972 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091
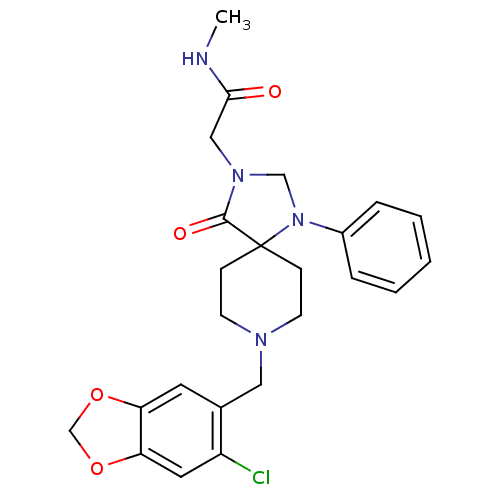
(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094
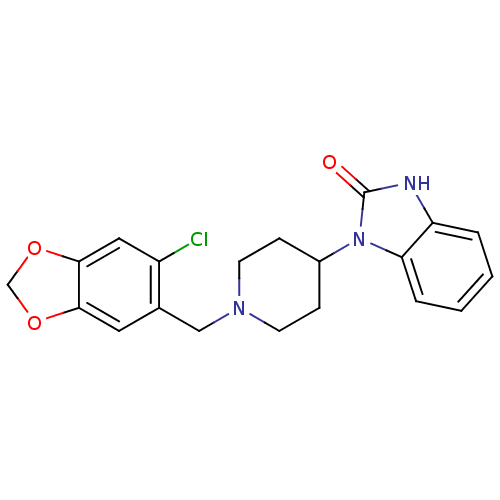
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080
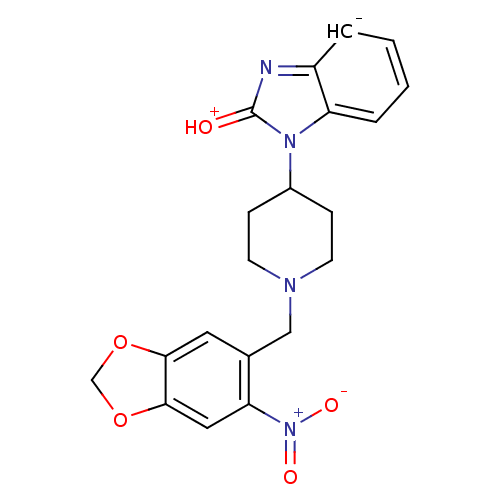
(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072
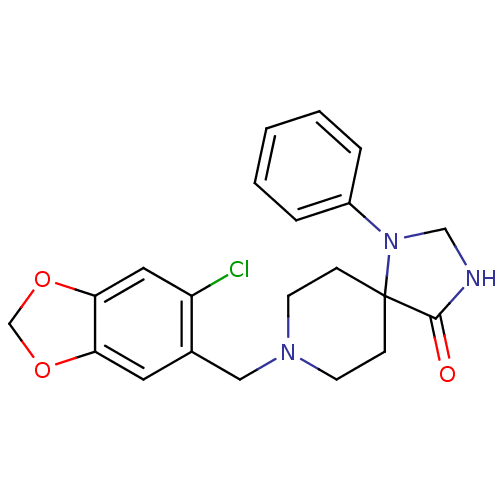
(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105111
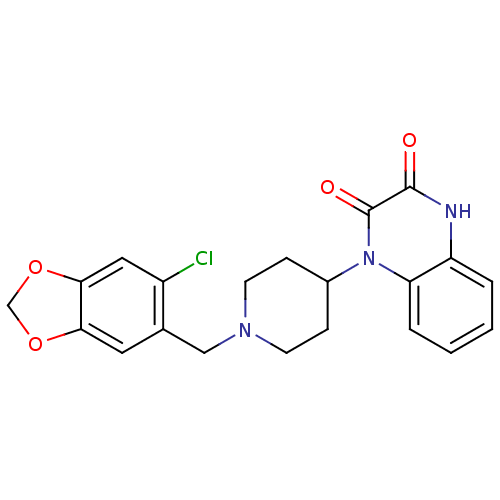
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c(=O)c1=O Show InChI InChI=1S/C21H20ClN3O4/c22-15-10-19-18(28-12-29-19)9-13(15)11-24-7-5-14(6-8-24)25-17-4-2-1-3-16(17)23-20(26)21(25)27/h1-4,9-10,14H,5-8,11-12H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of ligand binding to human delta opioid receptor. |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105106
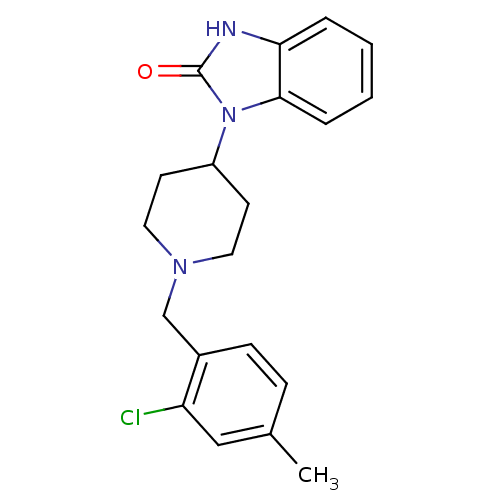
(1-[1-(2-Chloro-4-methyl-benzyl)-piperidin-4-yl]-1,...)Show SMILES Cc1ccc(CN2CCC(CC2)n2c3ccccc3[nH]c2=O)c(Cl)c1 Show InChI InChI=1S/C20H22ClN3O/c1-14-6-7-15(17(21)12-14)13-23-10-8-16(9-11-23)24-19-5-3-2-4-18(19)22-20(24)25/h2-7,12,16H,8-11,13H2,1H3,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105089
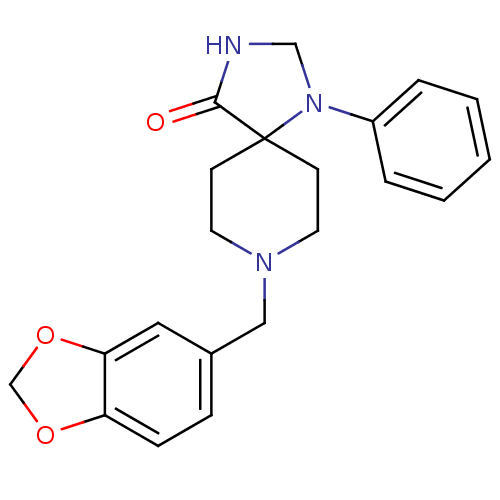
(8-Benzo[1,3]dioxol-5-ylmethyl-1-phenyl-1,3,8-triaz...)Show InChI InChI=1S/C21H23N3O3/c25-20-21(24(14-22-20)17-4-2-1-3-5-17)8-10-23(11-9-21)13-16-6-7-18-19(12-16)27-15-26-18/h1-7,12H,8-11,13-15H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105086
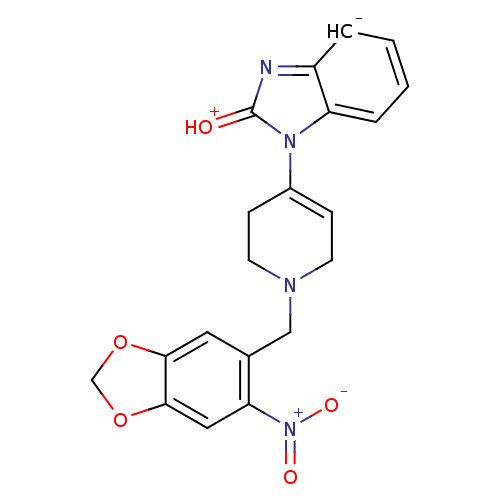
(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-1,2,3,6...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(=CC1)n1[c-]2ccccc2nc1=[OH+] |c:18| Show InChI InChI=1S/C20H17N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-5,9-10H,6-8,11-12H2/q-1/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human ORL1 orphanin receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50105088
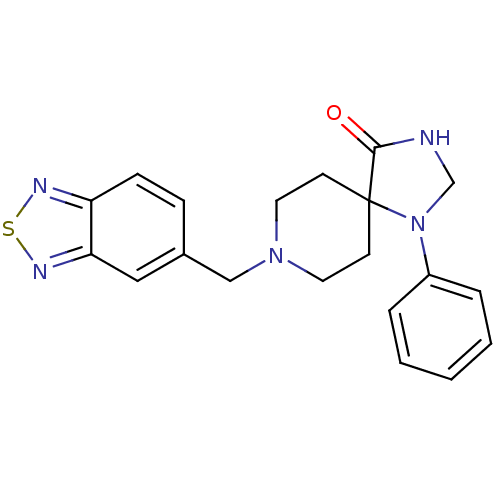
(1-Phenyl-8-[1,3,4]thiadiazolo[3,4-a]pyridazin-6-yl...)Show InChI InChI=1S/C20H21N5OS/c26-19-20(25(14-21-19)16-4-2-1-3-5-16)8-10-24(11-9-20)13-15-6-7-17-18(12-15)23-27-22-17/h1-7,12H,8-11,13-14H2,(H,21,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D3 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105088

(1-Phenyl-8-[1,3,4]thiadiazolo[3,4-a]pyridazin-6-yl...)Show InChI InChI=1S/C20H21N5OS/c26-19-20(25(14-21-19)16-4-2-1-3-5-16)8-10-24(11-9-20)13-15-6-7-17-18(12-15)23-27-22-17/h1-7,12H,8-11,13-14H2,(H,21,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105111

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c(=O)c1=O Show InChI InChI=1S/C21H20ClN3O4/c22-15-10-19-18(28-12-29-19)9-13(15)11-24-7-5-14(6-8-24)25-17-4-2-1-3-16(17)23-20(26)21(25)27/h1-4,9-10,14H,5-8,11-12H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080

(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of ligand binding to human delta opioid receptor. |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human ORL1 orphanin receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105089

(8-Benzo[1,3]dioxol-5-ylmethyl-1-phenyl-1,3,8-triaz...)Show InChI InChI=1S/C21H23N3O3/c25-20-21(24(14-22-20)17-4-2-1-3-5-17)8-10-23(11-9-21)13-16-6-7-18-19(12-16)27-15-26-18/h1-7,12H,8-11,13-15H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105076
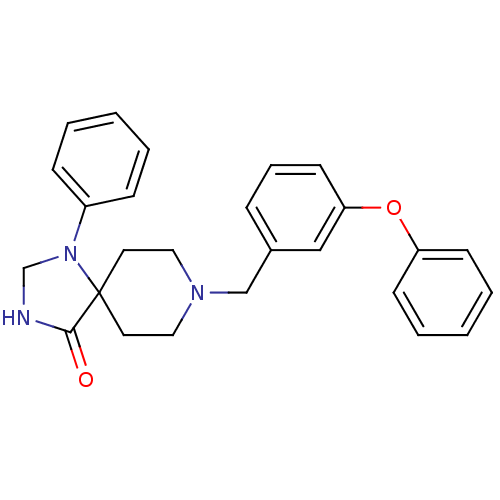
(8-(3-Phenoxy-benzyl)-1-phenyl-1,3,8-triaza-spiro[4...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(Cc2cccc(Oc3ccccc3)c2)CC1 Show InChI InChI=1S/C26H27N3O2/c30-25-26(29(20-27-25)22-9-3-1-4-10-22)14-16-28(17-15-26)19-21-8-7-13-24(18-21)31-23-11-5-2-6-12-23/h1-13,18H,14-17,19-20H2,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human ORL1 orphanin receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105076

(8-(3-Phenoxy-benzyl)-1-phenyl-1,3,8-triaza-spiro[4...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(Cc2cccc(Oc3ccccc3)c2)CC1 Show InChI InChI=1S/C26H27N3O2/c30-25-26(29(20-27-25)22-9-3-1-4-10-22)14-16-28(17-15-26)19-21-8-7-13-24(18-21)31-23-11-5-2-6-12-23/h1-13,18H,14-17,19-20H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105088

(1-Phenyl-8-[1,3,4]thiadiazolo[3,4-a]pyridazin-6-yl...)Show InChI InChI=1S/C20H21N5OS/c26-19-20(25(14-21-19)16-4-2-1-3-5-16)8-10-24(11-9-20)13-15-6-7-17-18(12-15)23-27-22-17/h1-7,12H,8-11,13-14H2,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of ligand binding to human delta opioid receptor. |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50105088

(1-Phenyl-8-[1,3,4]thiadiazolo[3,4-a]pyridazin-6-yl...)Show InChI InChI=1S/C20H21N5OS/c26-19-20(25(14-21-19)16-4-2-1-3-5-16)8-10-24(11-9-20)13-15-6-7-17-18(12-15)23-27-22-17/h1-7,12H,8-11,13-14H2,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D2 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of ligand binding to human delta opioid receptor. |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105061
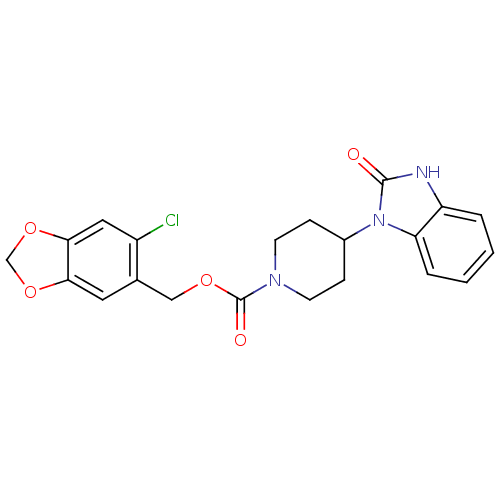
(4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidin...)Show SMILES Clc1cc2OCOc2cc1COC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C21H20ClN3O5/c22-15-10-19-18(29-12-30-19)9-13(15)11-28-21(27)24-7-5-14(6-8-24)25-17-4-2-1-3-16(17)23-20(25)26/h1-4,9-10,14H,5-8,11-12H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50105076

(8-(3-Phenoxy-benzyl)-1-phenyl-1,3,8-triaza-spiro[4...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(Cc2cccc(Oc3ccccc3)c2)CC1 Show InChI InChI=1S/C26H27N3O2/c30-25-26(29(20-27-25)22-9-3-1-4-10-22)14-16-28(17-15-26)19-21-8-7-13-24(18-21)31-23-11-5-2-6-12-23/h1-13,18H,14-17,19-20H2,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D3 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50105106

(1-[1-(2-Chloro-4-methyl-benzyl)-piperidin-4-yl]-1,...)Show SMILES Cc1ccc(CN2CCC(CC2)n2c3ccccc3[nH]c2=O)c(Cl)c1 Show InChI InChI=1S/C20H22ClN3O/c1-14-6-7-15(17(21)12-14)13-23-10-8-16(9-11-23)24-19-5-3-2-4-18(19)22-20(24)25/h2-7,12,16H,8-11,13H2,1H3,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D3 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50105089

(8-Benzo[1,3]dioxol-5-ylmethyl-1-phenyl-1,3,8-triaz...)Show InChI InChI=1S/C21H23N3O3/c25-20-21(24(14-22-20)17-4-2-1-3-5-17)8-10-23(11-9-21)13-16-6-7-18-19(12-16)27-15-26-18/h1-7,12H,8-11,13-15H2,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D3 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50105070
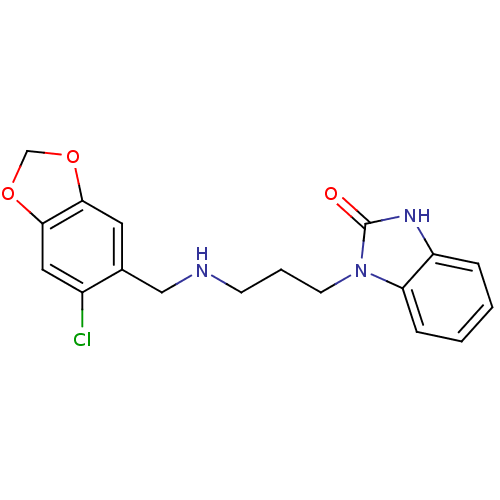
(1-{3-[(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-amino...)Show InChI InChI=1S/C18H18ClN3O3/c19-13-9-17-16(24-11-25-17)8-12(13)10-20-6-3-7-22-15-5-2-1-4-14(15)21-18(22)23/h1-2,4-5,8-9,20H,3,6-7,10-11H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of alpha-1 adrenergic receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105090
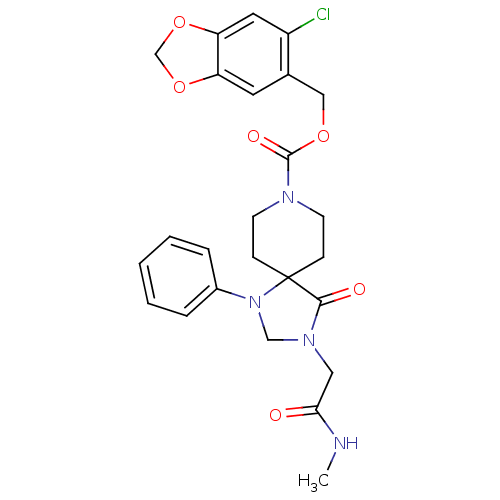
(3-Methylcarbamoylmethyl-4-oxo-1-phenyl-1,3,8-triaz...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)OCc2cc3OCOc3cc2Cl)C1=O Show InChI InChI=1S/C25H27ClN4O6/c1-27-22(31)13-29-15-30(18-5-3-2-4-6-18)25(23(29)32)7-9-28(10-8-25)24(33)34-14-17-11-20-21(12-19(17)26)36-16-35-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,27,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50105109
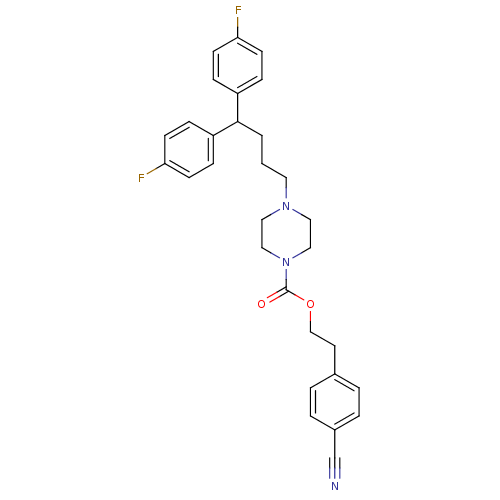
(4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...)Show SMILES Fc1ccc(cc1)C(CCCN1CCN(CC1)C(=O)OCCc1ccc(cc1)C#N)c1ccc(F)cc1 Show InChI InChI=1S/C30H31F2N3O2/c31-27-11-7-25(8-12-27)29(26-9-13-28(32)14-10-26)2-1-16-34-17-19-35(20-18-34)30(36)37-21-15-23-3-5-24(22-33)6-4-23/h3-14,29H,1-2,15-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D3 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105117
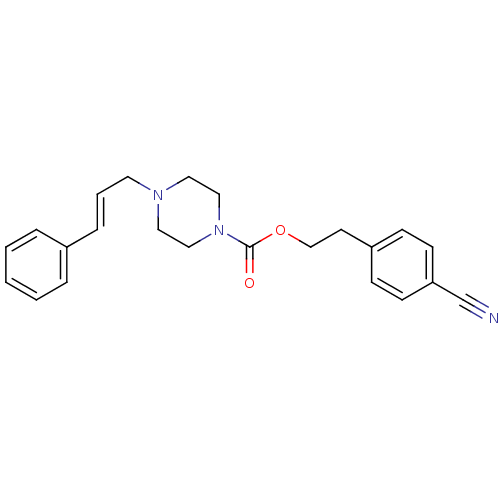
(4-(3-Phenyl-allyl)-piperazine-1-carboxylic acid 2-...)Show SMILES O=C(OCCc1ccc(cc1)C#N)N1CCN(C\C=C\c2ccccc2)CC1 Show InChI InChI=1S/C23H25N3O2/c24-19-22-10-8-21(9-11-22)12-18-28-23(27)26-16-14-25(15-17-26)13-4-7-20-5-2-1-3-6-20/h1-11H,12-18H2/b7-4+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50105102
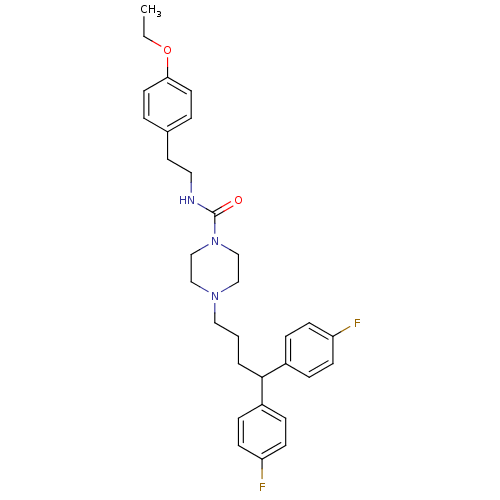
(4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...)Show SMILES CCOc1ccc(CCNC(=O)N2CCN(CCCC(c3ccc(F)cc3)c3ccc(F)cc3)CC2)cc1 Show InChI InChI=1S/C31H37F2N3O2/c1-2-38-29-15-5-24(6-16-29)17-18-34-31(37)36-22-20-35(21-23-36)19-3-4-30(25-7-11-27(32)12-8-25)26-9-13-28(33)14-10-26/h5-16,30H,2-4,17-23H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human 5-hydroxytryptamine 2A receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105070

(1-{3-[(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-amino...)Show InChI InChI=1S/C18H18ClN3O3/c19-13-9-17-16(24-11-25-17)8-12(13)10-20-6-3-7-22-15-5-2-1-4-14(15)21-18(22)23/h1-2,4-5,8-9,20H,3,6-7,10-11H2,(H,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080

(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of ligand binding to human delta opioid receptor. |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105106

(1-[1-(2-Chloro-4-methyl-benzyl)-piperidin-4-yl]-1,...)Show SMILES Cc1ccc(CN2CCC(CC2)n2c3ccccc3[nH]c2=O)c(Cl)c1 Show InChI InChI=1S/C20H22ClN3O/c1-14-6-7-15(17(21)12-14)13-23-10-8-16(9-11-23)24-19-5-3-2-4-18(19)22-20(24)25/h2-7,12,16H,8-11,13H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50105110
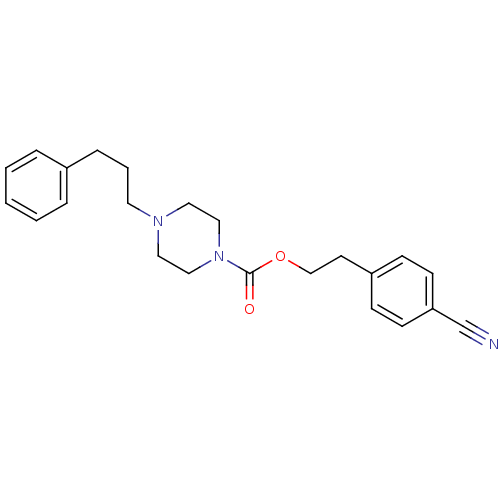
(4-(3-Phenyl-propyl)-piperazine-1-carboxylic acid 2...)Show InChI InChI=1S/C23H27N3O2/c24-19-22-10-8-21(9-11-22)12-18-28-23(27)26-16-14-25(15-17-26)13-4-7-20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50105107
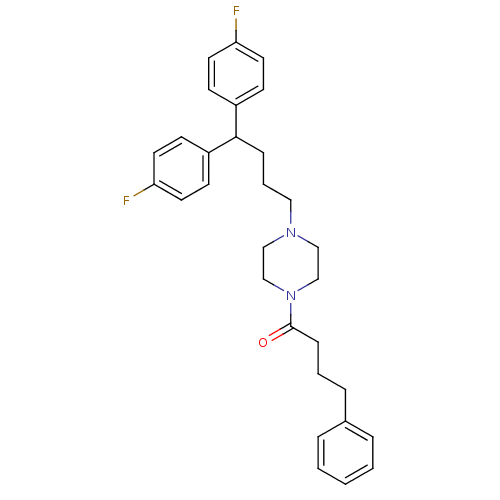
(1-{4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazin-1...)Show SMILES Fc1ccc(cc1)C(CCCN1CCN(CC1)C(=O)CCCc1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C30H34F2N2O/c31-27-15-11-25(12-16-27)29(26-13-17-28(32)18-14-26)9-5-19-33-20-22-34(23-21-33)30(35)10-4-8-24-6-2-1-3-7-24/h1-3,6-7,11-18,29H,4-5,8-10,19-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D4.4 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human ORL1 orphanin receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S
(Homo sapiens (Human)) | BDBM50105105
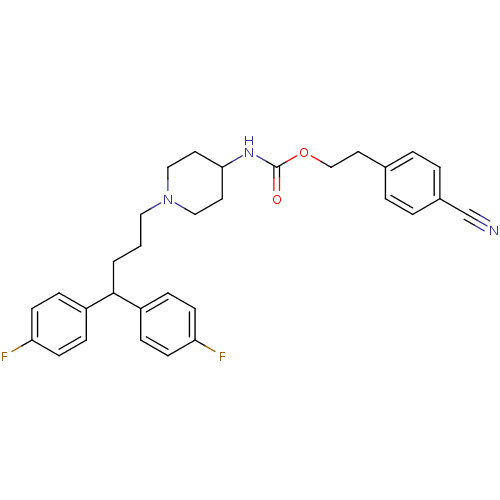
(CHEMBL116463 | {1-[4,4-Bis-(4-fluoro-phenyl)-butyl...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)NC(=O)OCCc1ccc(cc1)C#N)c1ccc(F)cc1 Show InChI InChI=1S/C31H33F2N3O2/c32-27-11-7-25(8-12-27)30(26-9-13-28(33)14-10-26)2-1-18-36-19-15-29(16-20-36)35-31(37)38-21-17-23-3-5-24(22-34)6-4-23/h3-14,29-30H,1-2,15-21H2,(H,35,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against L-type calcium channel verapamil site |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105061

(4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidin...)Show SMILES Clc1cc2OCOc2cc1COC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C21H20ClN3O5/c22-15-10-19-18(29-12-30-19)9-13(15)11-28-21(27)24-7-5-14(6-8-24)25-17-4-2-1-3-16(17)23-20(25)26/h1-4,9-10,14H,5-8,11-12H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105086

(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-1,2,3,6...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(=CC1)n1[c-]2ccccc2nc1=[OH+] |c:18| Show InChI InChI=1S/C20H17N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-5,9-10H,6-8,11-12H2/q-1/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50105070

(1-{3-[(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-amino...)Show InChI InChI=1S/C18H18ClN3O3/c19-13-9-17-16(24-11-25-17)8-12(13)10-20-6-3-7-22-15-5-2-1-4-14(15)21-18(22)23/h1-2,4-5,8-9,20H,3,6-7,10-11H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of sigma receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105090

(3-Methylcarbamoylmethyl-4-oxo-1-phenyl-1,3,8-triaz...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(CC2)C(=O)OCc2cc3OCOc3cc2Cl)C1=O Show InChI InChI=1S/C25H27ClN4O6/c1-27-22(31)13-29-15-30(18-5-3-2-4-6-18)25(23(29)32)7-9-28(10-8-25)24(33)34-14-17-11-20-21(12-19(17)26)36-16-35-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50105101
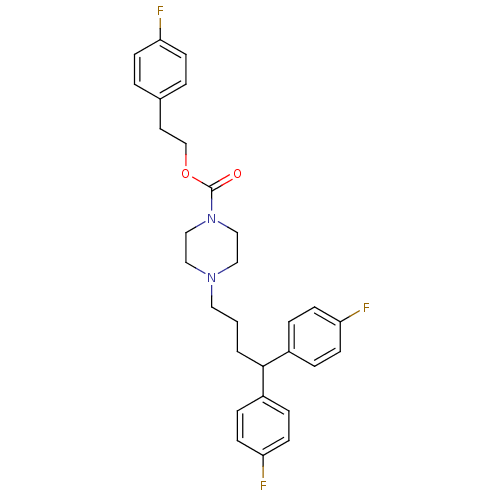
(4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...)Show SMILES Fc1ccc(CCOC(=O)N2CCN(CCCC(c3ccc(F)cc3)c3ccc(F)cc3)CC2)cc1 Show InChI InChI=1S/C29H31F3N2O2/c30-25-9-3-22(4-10-25)15-21-36-29(35)34-19-17-33(18-20-34)16-1-2-28(23-5-11-26(31)12-6-23)24-7-13-27(32)14-8-24/h3-14,28H,1-2,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D4.4 |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data