

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775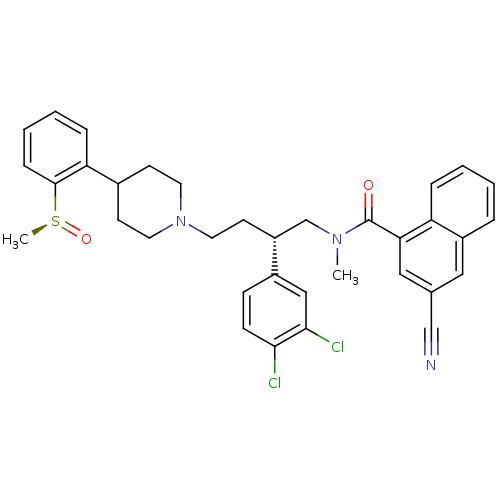 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118098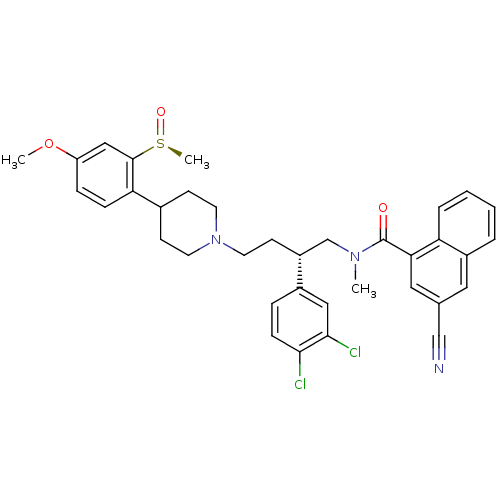 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118100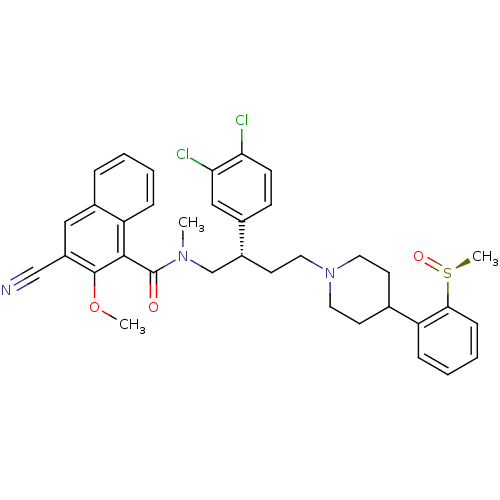 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118099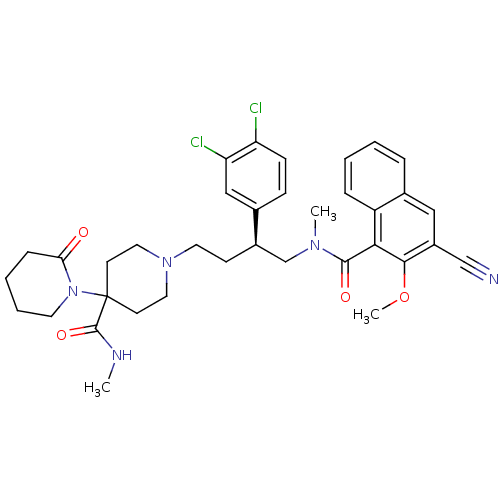 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50409575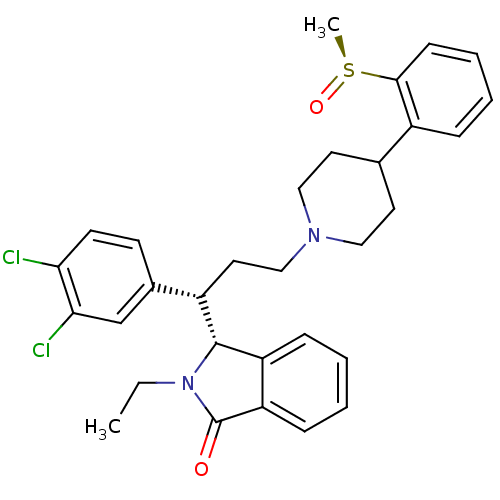 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118099 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118099 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118100 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118100 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50409575 (CHEMBL339767 | ZD-7944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to human Tachykinin receptor 1 expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50409575 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||