

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50103430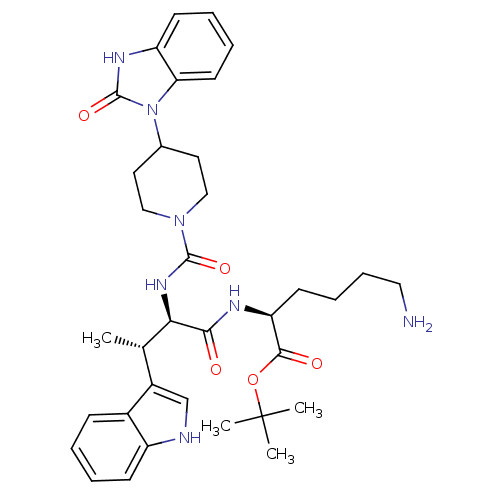 (6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Somatostatin receptor type 2 (hsst2) | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50103430 (6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Somatostatin receptor type 3 (hsst3) | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50103430 (6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Somatostatin receptor type 4 (hsst4) | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50103430 (6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Somatostatin receptor type 5 (hsst5) | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50121929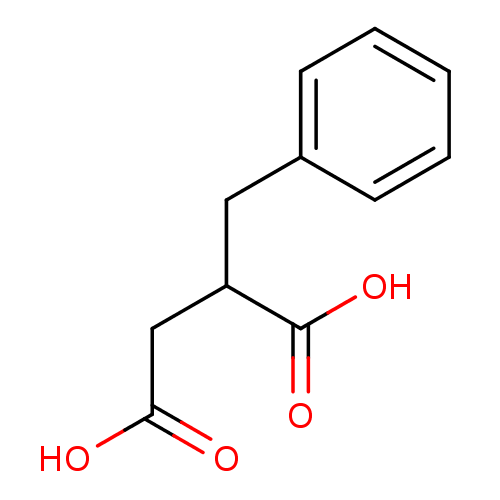 ((phenylmethyl)butanedioic acid | 2-benzylbutanedio...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of Carboxypeptidase A | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50103430 (6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Somatostatin receptor type 1 (hsst1) | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50121917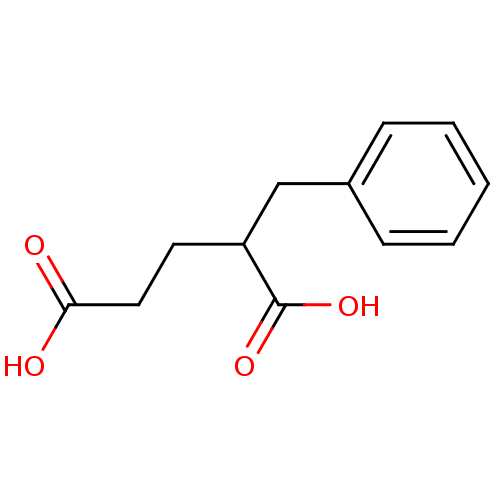 (2-Benzyl-pentanedioic acid | CHEMBL153232) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of Carboxypeptidase A | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50121928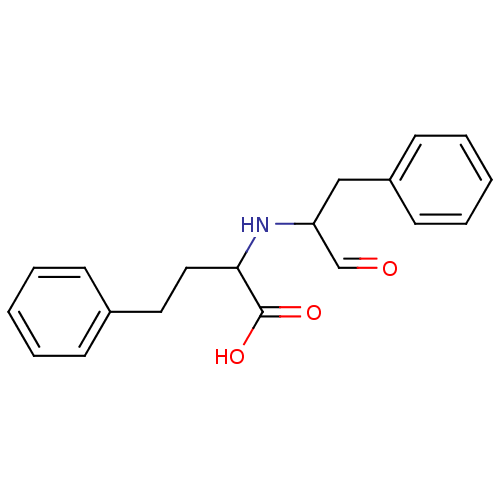 (2-(1-Formyl-2-phenyl-ethylamino)-4-phenyl-butyric ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) in venom of Bothrops jararaca | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50049478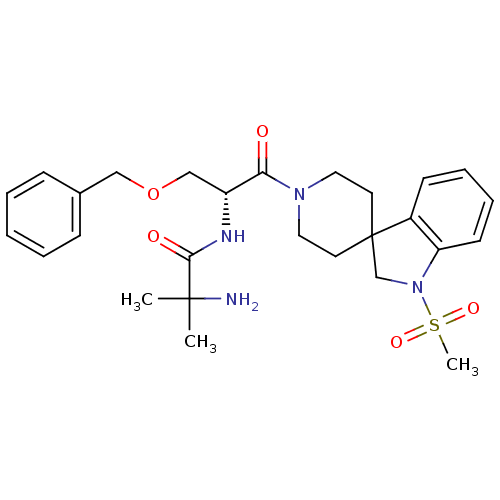 (1-{[(2R)-3-(benzyloxy)-1-{1-methanesulfonyl-1,2-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) receptor expressed in COS-7 cells | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50121930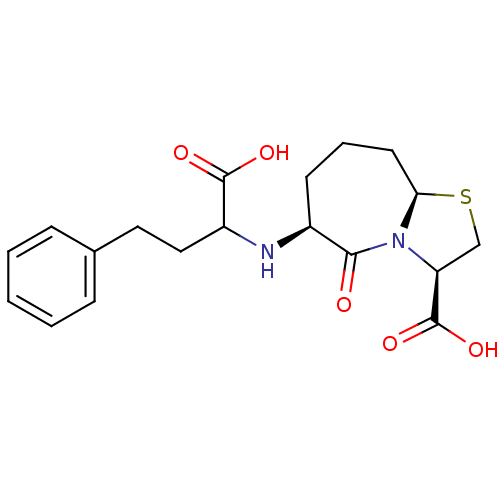 (6-(1-Carboxy-3-phenyl-propylamino)-5-oxo-octahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50121927 (CHEMBL437713 | Glu-Ser-OctSer-phe-Leu-Ser-Pro-Glu-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) expressed in COS-7 cells | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50367879 (LISINOPRIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50367254 (ENALAPRILAT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM81962 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Cholecystokinin type B receptor induced guinea pig gall bladder contractions when given intravenously | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50121916 (CHEMBL433540 | His-D-Trp-Ala-Trp-D-Phe-Lys-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) expressed in COS-7 cells | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50011367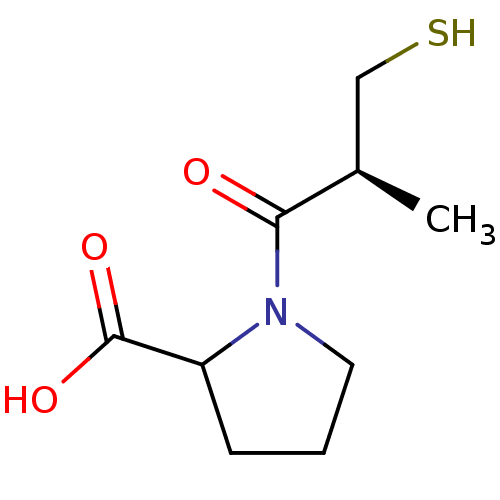 (1-(3-Mercapto-2-methyl-propionyl)-pyrrolidine-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50121926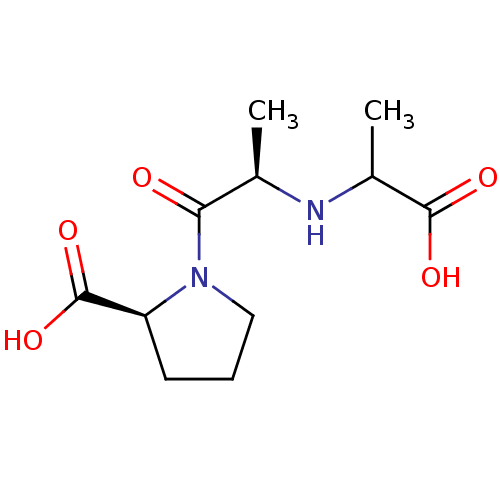 (1-[2-(1-Carboxy-ethylamino)-propionyl]-pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Cholecystokinin type B receptor induced guinea pig gall bladder contractions when given intravenously | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM81962 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50121922 (7-(9-Hydroxy-2-isobutyl-2-methyl-3-oxo-2,3,9,9a-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50044234 (1-[2-(1-Carboxy-ethylamino)-propionyl]-pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50027357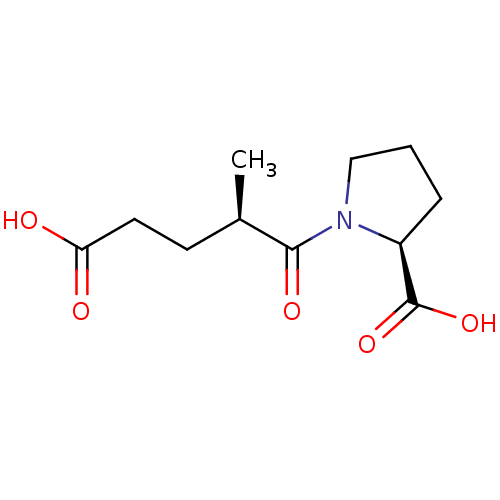 (1-(4-Carboxy-2-methyl-butyryl)-pyrrolidine-2-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50027757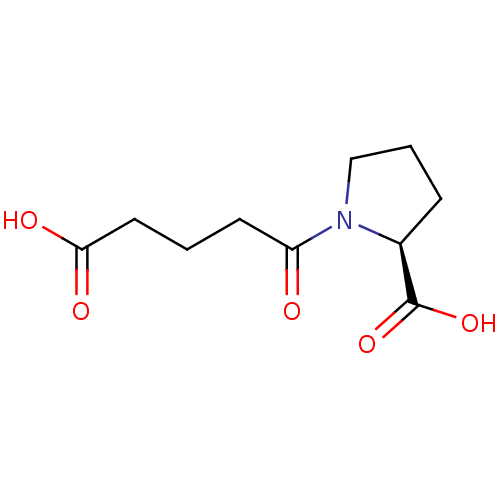 (1-(4-Carboxy-butyryl)-pyrrolidine-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50121922 (7-(9-Hydroxy-2-isobutyl-2-methyl-3-oxo-2,3,9,9a-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Cholecystokinin type B receptor induced guinea pig gall bladder contractions when given intravenously | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50121925 (1-(4-Carboxy-pentanoyl)-pyrrolidine-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venom | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||