 Found 713 hits Enz. Inhib. hit(s) with all data for entry = 50037119
Found 713 hits Enz. Inhib. hit(s) with all data for entry = 50037119 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097289
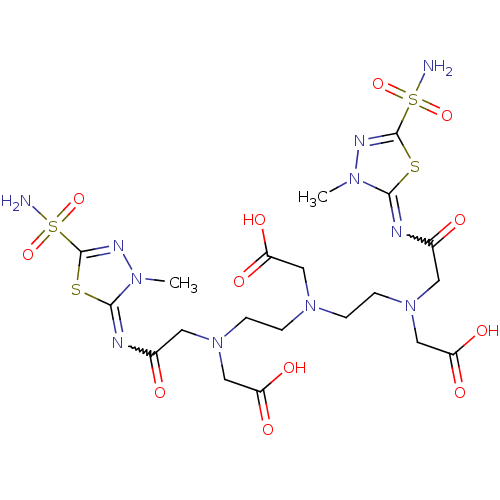
(((2-{Carboxymethyl-[2-(carboxymethyl-{[3-methyl-5-...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)CC(O)=O)S(N)(=O)=O |w:24.24,6.7| Show InChI InChI=1S/C20H31N11O12S4/c1-27-17(44-19(25-27)46(21,40)41)23-12(32)7-30(10-15(36)37)5-3-29(9-14(34)35)4-6-31(11-16(38)39)8-13(33)24-18-28(2)26-20(45-18)47(22,42)43/h3-11H2,1-2H3,(H,34,35)(H,36,37)(H,38,39)(H2,21,40,41)(H2,22,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097275
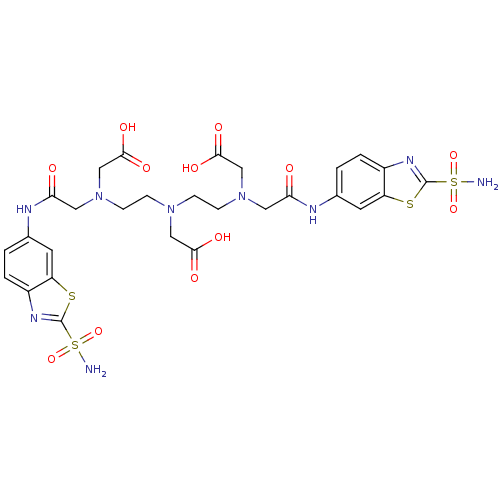
(CHEMBL286586 | {{2-[Carboxymethyl-(2-{carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H33N9O12S4/c29-52(46,47)27-33-18-3-1-16(9-20(18)50-27)31-22(38)11-36(14-25(42)43)7-5-35(13-24(40)41)6-8-37(15-26(44)45)12-23(39)32-17-2-4-19-21(10-17)51-28(34-19)53(30,48)49/h1-4,9-10H,5-8,11-15H2,(H,31,38)(H,32,39)(H,40,41)(H,42,43)(H,44,45)(H2,29,46,47)(H2,30,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097307
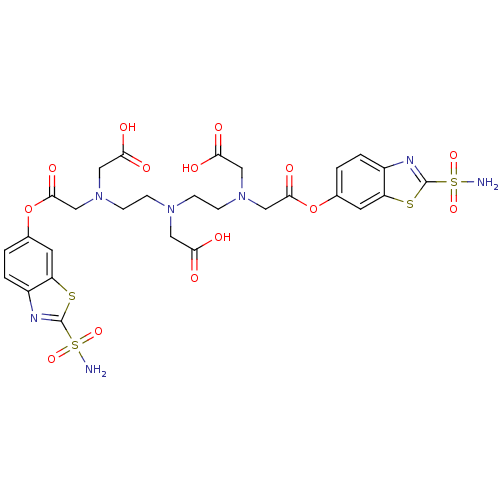
(CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H31N7O14S4/c29-52(44,45)27-31-18-3-1-16(9-20(18)50-27)48-25(42)14-34(12-23(38)39)7-5-33(11-22(36)37)6-8-35(13-24(40)41)15-26(43)49-17-2-4-19-21(10-17)51-28(32-19)53(30,46)47/h1-4,9-10H,5-8,11-15H2,(H,36,37)(H,38,39)(H,40,41)(H2,29,44,45)(H2,30,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292154
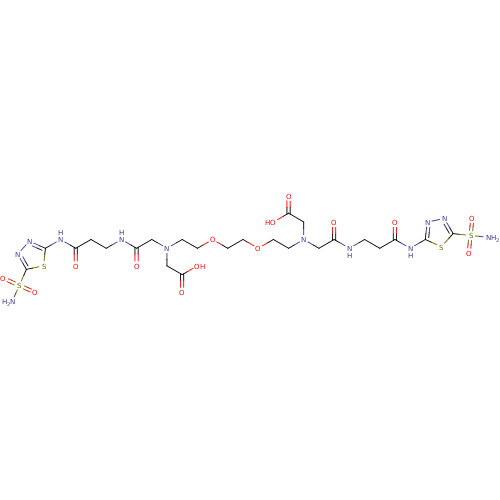
(((2-{2-[2-(Carboxymethyl-{[2-(5-sulfamoyl-[1,3,4]t...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C24H38N12O14S4/c25-53(45,46)23-33-31-21(51-23)29-15(37)1-3-27-17(39)11-35(13-19(41)42)5-7-49-9-10-50-8-6-36(14-20(43)44)12-18(40)28-4-2-16(38)30-22-32-34-24(52-22)54(26,47)48/h1-14H2,(H,27,39)(H,28,40)(H,41,42)(H,43,44)(H2,25,45,46)(H2,26,47,48)(H,29,31,37)(H,30,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292100

(CHEMBL284354 | {{2-[2-(2-{Carboxymethyl-[(2-sulfam...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H34N8O12S4/c29-51(43,44)27-33-19-3-1-17(11-21(19)49-27)31-23(37)13-35(15-25(39)40)5-7-47-9-10-48-8-6-36(16-26(41)42)14-24(38)32-18-2-4-20-22(12-18)50-28(34-20)52(30,45)46/h1-4,11-12H,5-10,13-16H2,(H,31,37)(H,32,38)(H,39,40)(H,41,42)(H2,29,43,44)(H2,30,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097288
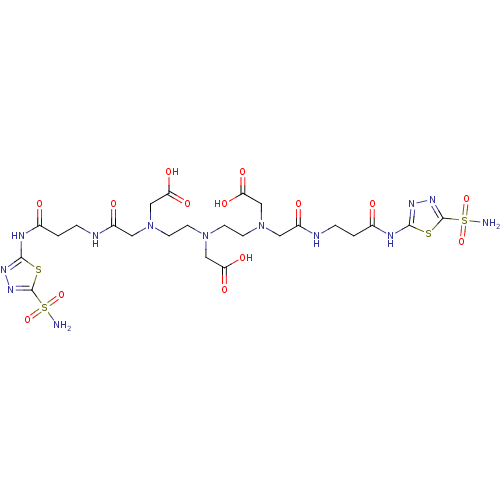
(((2-{Carboxymethyl-[2-(carboxymethyl-{[2-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C24H37N13O14S4/c25-54(48,49)23-33-31-21(52-23)29-14(38)1-3-27-16(40)9-36(12-19(44)45)7-5-35(11-18(42)43)6-8-37(13-20(46)47)10-17(41)28-4-2-15(39)30-22-32-34-24(53-22)55(26,50)51/h1-13H2,(H,27,40)(H,28,41)(H,42,43)(H,44,45)(H,46,47)(H2,25,48,49)(H2,26,50,51)(H,29,31,38)(H,30,32,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292022
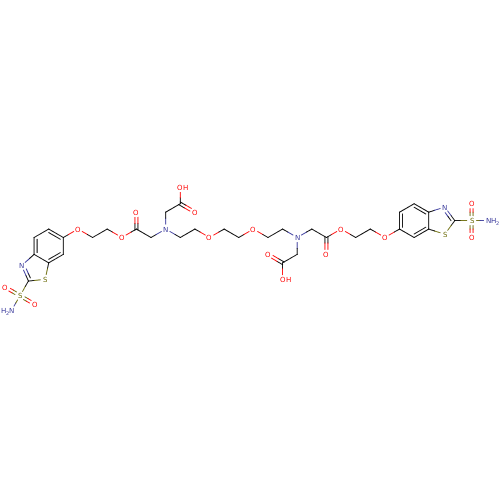
(CHEMBL35186 | {{2-[2-(2-{Carboxymethyl-[2-(2-sulfa...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)OCCOc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C32H40N6O16S4/c33-57(45,46)31-35-23-3-1-21(15-25(23)55-31)51-11-13-53-29(43)19-37(17-27(39)40)5-7-49-9-10-50-8-6-38(18-28(41)42)20-30(44)54-14-12-52-22-2-4-24-26(16-22)56-32(36-24)58(34,47)48/h1-4,15-16H,5-14,17-20H2,(H,39,40)(H,41,42)(H2,33,45,46)(H2,34,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097293
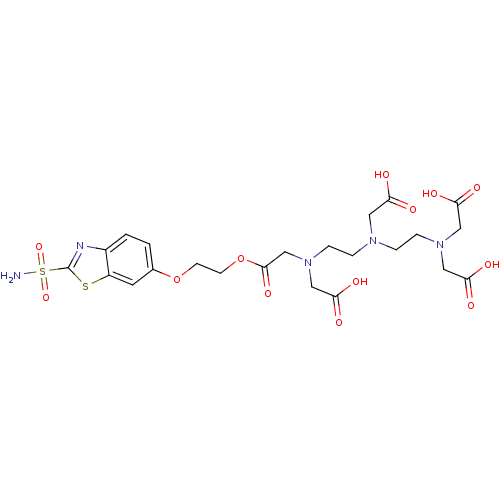
(CHEMBL34852 | {(2-{[2-(Bis-carboxymethyl-amino)-et...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C23H31N5O13S2/c24-43(38,39)23-25-16-2-1-15(9-17(16)42-23)40-7-8-41-22(37)14-28(13-21(35)36)6-4-26(10-18(29)30)3-5-27(11-19(31)32)12-20(33)34/h1-2,9H,3-8,10-14H2,(H,29,30)(H,31,32)(H,33,34)(H,35,36)(H2,24,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097307

(CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H31N7O14S4/c29-52(44,45)27-31-18-3-1-16(9-20(18)50-27)48-25(42)14-34(12-23(38)39)7-5-33(11-22(36)37)6-8-35(13-24(40)41)15-26(43)49-17-2-4-19-21(10-17)51-28(32-19)53(30,46)47/h1-4,9-10H,5-8,11-15H2,(H,36,37)(H,38,39)(H,40,41)(H2,29,44,45)(H2,30,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292077
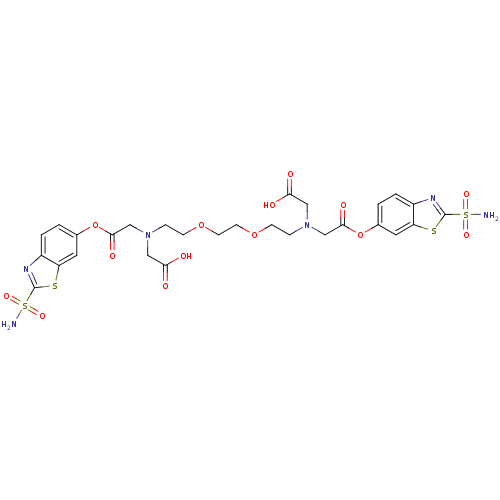
(CHEMBL33478 | [[2-(2-{2-[Carboxymethyl-(2-sulfamoy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H32N6O14S4/c29-51(41,42)27-31-19-3-1-17(11-21(19)49-27)47-25(39)15-33(13-23(35)36)5-7-45-9-10-46-8-6-34(14-24(37)38)16-26(40)48-18-2-4-20-22(12-18)50-28(32-20)52(30,43)44/h1-4,11-12H,5-10,13-16H2,(H,35,36)(H,37,38)(H2,29,41,42)(H2,30,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292007
(CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C18H28N10O12S4/c19-43(35,36)17-25-23-15(41-17)21-11(29)7-27(9-13(31)32)1-3-39-5-6-40-4-2-28(10-14(33)34)8-12(30)22-16-24-26-18(42-16)44(20,37)38/h1-10H2,(H,31,32)(H,33,34)(H2,19,35,36)(H2,20,37,38)(H,21,23,29)(H,22,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292023
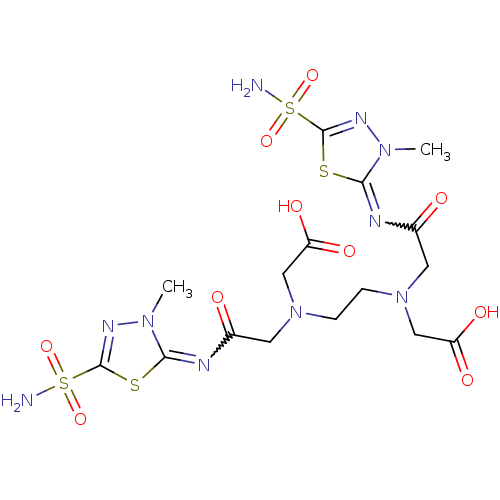
(CHEMBL1795060 | CHEMBL35495 | {(2-{Carboxymethyl-[...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)S(N)(=O)=O |w:21.21,6.7| Show InChI InChI=1S/C16H24N10O10S4/c1-23-13(37-15(21-23)39(17,33)34)19-9(27)5-25(7-11(29)30)3-4-26(8-12(31)32)6-10(28)20-14-24(2)22-16(38-14)40(18,35)36/h3-8H2,1-2H3,(H,29,30)(H,31,32)(H2,17,33,34)(H2,18,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292008
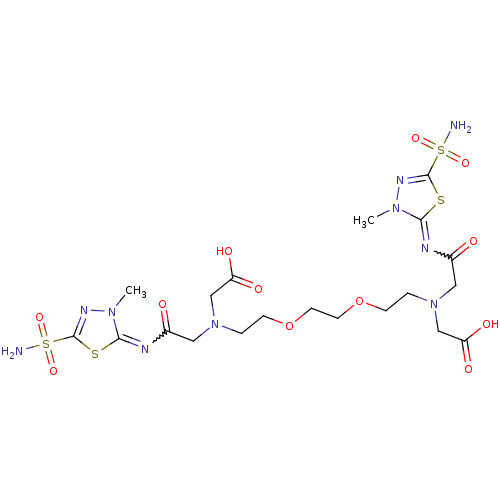
(CHEMBL1795065 | CHEMBL35272 | {{2-[2-(2-{Carboxyme...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)S(N)(=O)=O |w:27.27,6.7| Show InChI InChI=1S/C20H32N10O12S4/c1-27-17(43-19(25-27)45(21,37)38)23-13(31)9-29(11-15(33)34)3-5-41-7-8-42-6-4-30(12-16(35)36)10-14(32)24-18-28(2)26-20(44-18)46(22,39)40/h3-12H2,1-2H3,(H,33,34)(H,35,36)(H2,21,37,38)(H2,22,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292132
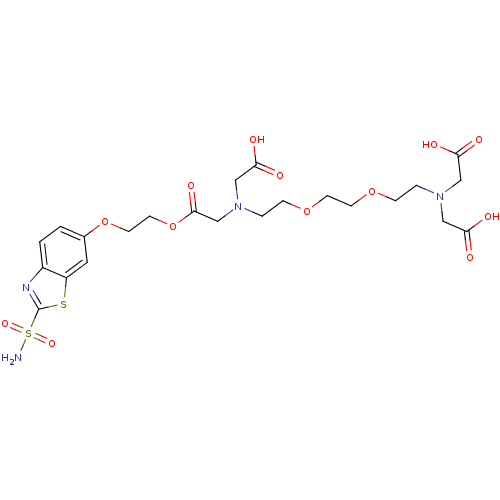
(CHEMBL286131 | {(2-{2-[2-(Bis-carboxymethyl-amino)...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C23H32N4O13S2/c24-42(35,36)23-25-17-2-1-16(11-18(17)41-23)39-9-10-40-22(34)15-27(14-21(32)33)4-6-38-8-7-37-5-3-26(12-19(28)29)13-20(30)31/h1-2,11H,3-10,12-15H2,(H,28,29)(H,30,31)(H,32,33)(H2,24,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097309
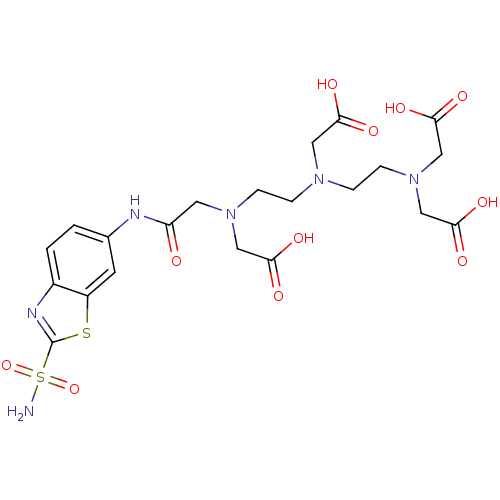
((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H28N6O11S2/c22-40(37,38)21-24-14-2-1-13(7-15(14)39-21)23-16(28)8-26(10-18(31)32)5-3-25(9-17(29)30)4-6-27(11-19(33)34)12-20(35)36/h1-2,7H,3-6,8-12H2,(H,23,28)(H,29,30)(H,31,32)(H,33,34)(H,35,36)(H2,22,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50228325
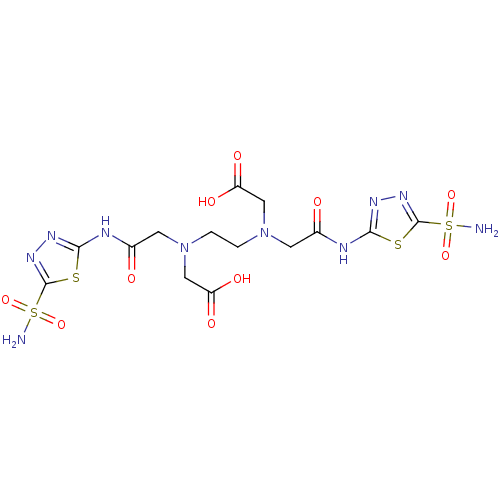
(CHEMBL1795059 | CHEMBL34546 | {(2-{Carboxymethyl-[...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C14H20N10O10S4/c15-37(31,32)13-21-19-11(35-13)17-7(25)3-23(5-9(27)28)1-2-24(6-10(29)30)4-8(26)18-12-20-22-14(36-12)38(16,33)34/h1-6H2,(H,27,28)(H,29,30)(H2,15,31,32)(H2,16,33,34)(H,17,19,25)(H,18,20,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097298
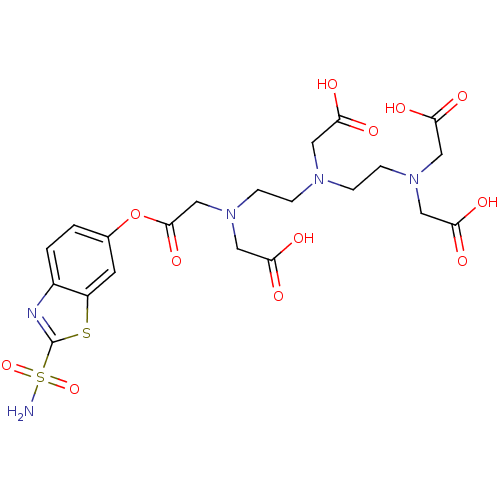
(CHEMBL34317 | [(2-{[2-(Bis-carboxymethyl-amino)-et...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H27N5O12S2/c22-40(36,37)21-23-14-2-1-13(7-15(14)39-21)38-20(35)12-26(11-19(33)34)6-4-24(8-16(27)28)3-5-25(9-17(29)30)10-18(31)32/h1-2,7H,3-6,8-12H2,(H,27,28)(H,29,30)(H,31,32)(H,33,34)(H2,22,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097300
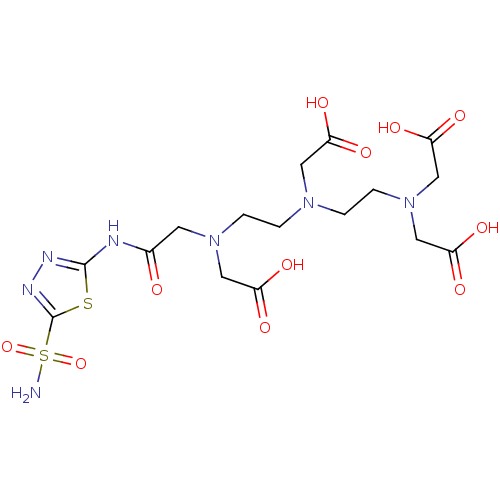
((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C16H25N7O11S2/c17-36(33,34)16-20-19-15(35-16)18-10(24)5-22(7-12(27)28)3-1-21(6-11(25)26)2-4-23(8-13(29)30)9-14(31)32/h1-9H2,(H,25,26)(H,27,28)(H,29,30)(H,31,32)(H2,17,33,34)(H,18,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292119
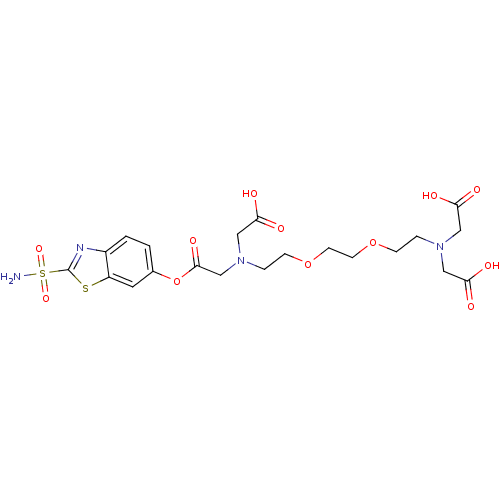
(CHEMBL284500 | [(2-{2-[2-(Bis-carboxymethyl-amino)...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H28N4O12S2/c22-39(33,34)21-23-15-2-1-14(9-16(15)38-21)37-20(32)13-25(12-19(30)31)4-6-36-8-7-35-5-3-24(10-17(26)27)11-18(28)29/h1-2,9H,3-8,10-13H2,(H,26,27)(H,28,29)(H,30,31)(H2,22,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292146

(CHEMBL35593 | {(2-{Carboxymethyl-[2-(2-sulfamoyl-b...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CC(O)=O)CC(=O)OCCOc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H32N6O14S4/c29-51(41,42)27-31-19-3-1-17(11-21(19)49-27)45-7-9-47-25(39)15-33(13-23(35)36)5-6-34(14-24(37)38)16-26(40)48-10-8-46-18-2-4-20-22(12-18)50-28(32-20)52(30,43)44/h1-4,11-12H,5-10,13-16H2,(H,35,36)(H,37,38)(H2,29,41,42)(H2,30,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292127
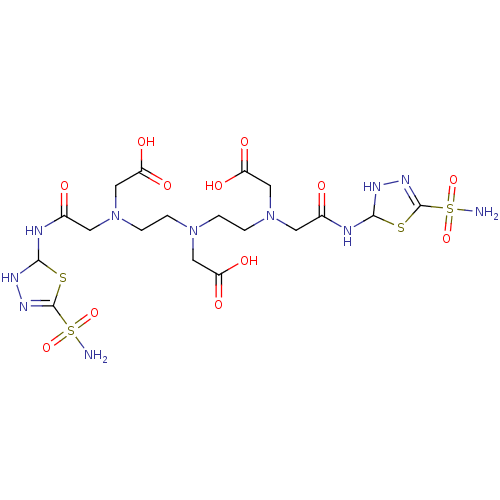
(CHEMBL262651 | {{2-[Carboxymethyl-(2-{carboxymethy...)Show SMILES NS(=O)(=O)C1=NNC(NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)NC2NN=C(S2)S(N)(=O)=O)CC(O)=O)CC(O)=O)S1 |c:29,t:4| Show InChI InChI=1S/C18H31N11O12S4/c19-44(38,39)17-25-23-15(42-17)21-10(30)5-28(8-13(34)35)3-1-27(7-12(32)33)2-4-29(9-14(36)37)6-11(31)22-16-24-26-18(43-16)45(20,40)41/h15-16,23-24H,1-9H2,(H,21,30)(H,22,31)(H,32,33)(H,34,35)(H,36,37)(H2,19,38,39)(H2,20,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292124
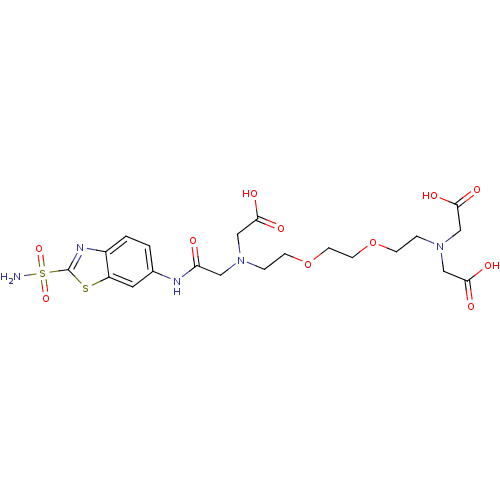
(CHEMBL35227 | {(2-{2-[2-(Bis-carboxymethyl-amino)-...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C21H29N5O11S2/c22-39(34,35)21-24-15-2-1-14(9-16(15)38-21)23-17(27)10-25(11-18(28)29)3-5-36-7-8-37-6-4-26(12-19(30)31)13-20(32)33/h1-2,9H,3-8,10-13H2,(H,23,27)(H,28,29)(H,30,31)(H,32,33)(H2,22,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097302
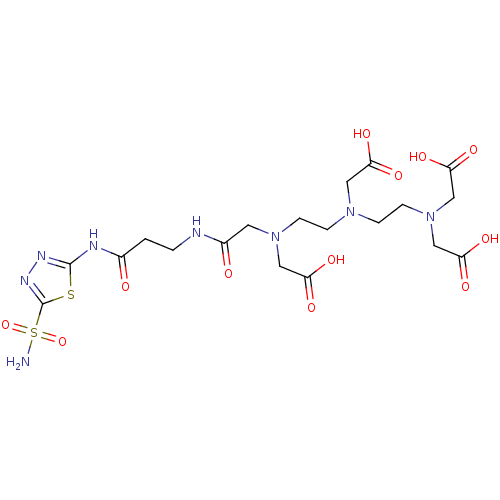
(CHEMBL416410 | [Carboxymethyl-(2-{carboxymethyl-[2...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C19H30N8O12S2/c20-41(38,39)19-24-23-18(40-19)22-12(28)1-2-21-13(29)7-26(9-15(32)33)5-3-25(8-14(30)31)4-6-27(10-16(34)35)11-17(36)37/h1-11H2,(H,21,29)(H,30,31)(H,32,33)(H,34,35)(H,36,37)(H2,20,38,39)(H,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292067
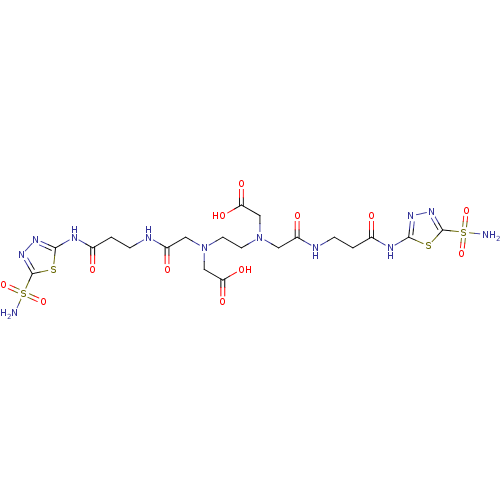
(([2-(Carboxymethyl-{[2-(5-sulfamoyl-[1,3,4]thiadia...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CC(O)=O)CC(=O)NCCC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C20H30N12O12S4/c21-47(41,42)19-29-27-17(45-19)25-11(33)1-3-23-13(35)7-31(9-15(37)38)5-6-32(10-16(39)40)8-14(36)24-4-2-12(34)26-18-28-30-20(46-18)48(22,43)44/h1-10H2,(H,23,35)(H,24,36)(H,37,38)(H,39,40)(H2,21,41,42)(H2,22,43,44)(H,25,27,33)(H,26,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292008

(CHEMBL1795065 | CHEMBL35272 | {{2-[2-(2-{Carboxyme...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)S(N)(=O)=O |w:27.27,6.7| Show InChI InChI=1S/C20H32N10O12S4/c1-27-17(43-19(25-27)45(21,37)38)23-13(31)9-29(11-15(33)34)3-5-41-7-8-42-6-4-30(12-16(35)36)10-14(32)24-18-28(2)26-20(44-18)46(22,39)40/h3-12H2,1-2H3,(H,33,34)(H,35,36)(H2,21,37,38)(H2,22,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292056
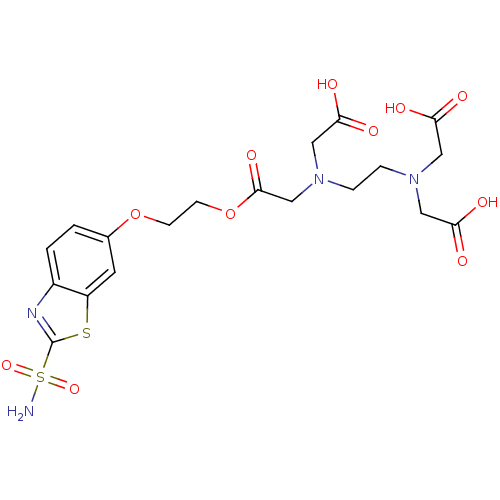
(CHEMBL414154 | [Carboxymethyl-(2-{carboxymethyl-[2...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C19H24N4O11S2/c20-36(31,32)19-21-13-2-1-12(7-14(13)35-19)33-5-6-34-18(30)11-23(10-17(28)29)4-3-22(8-15(24)25)9-16(26)27/h1-2,7H,3-6,8-11H2,(H,24,25)(H,26,27)(H,28,29)(H2,20,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
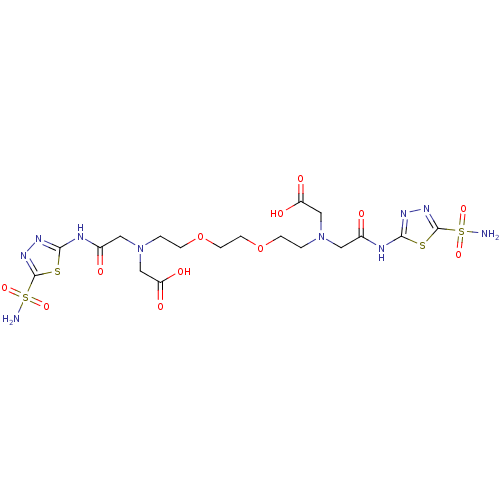
(Homo sapiens (Human)) | BDBM50097289

(((2-{Carboxymethyl-[2-(carboxymethyl-{[3-methyl-5-...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)N=c1sc(nn1C)S(N)(=O)=O)CC(O)=O)CC(O)=O)S(N)(=O)=O |w:24.24,6.7| Show InChI InChI=1S/C20H31N11O12S4/c1-27-17(44-19(25-27)46(21,40)41)23-12(32)7-30(10-15(36)37)5-3-29(9-14(34)35)4-6-31(11-16(38)39)8-13(33)24-18-28(2)26-20(45-18)47(22,42)43/h3-11H2,1-2H3,(H,34,35)(H,36,37)(H,38,39)(H2,21,40,41)(H2,22,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292133
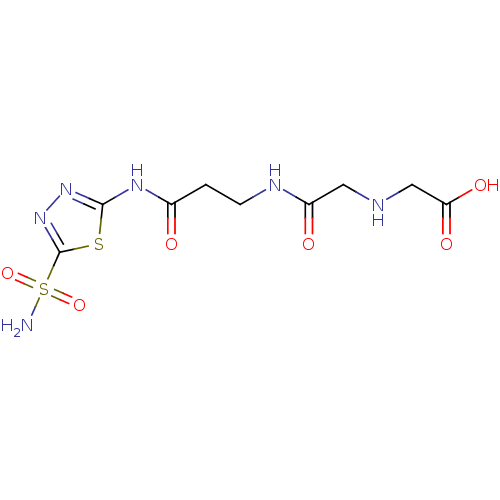
(({[2-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)...)Show InChI InChI=1S/C9H14N6O6S2/c10-23(20,21)9-15-14-8(22-9)13-5(16)1-2-12-6(17)3-11-4-7(18)19/h11H,1-4H2,(H,12,17)(H,18,19)(H2,10,20,21)(H,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292096

(CHEMBL36447 | [Carboxymethyl-(2-{2-[2-(carboxymeth...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C19H31N7O12S2/c20-40(35,36)19-24-23-18(39-19)22-13(27)1-2-21-14(28)9-25(10-15(29)30)3-5-37-7-8-38-6-4-26(11-16(31)32)12-17(33)34/h1-12H2,(H,21,28)(H,29,30)(H,31,32)(H,33,34)(H2,20,35,36)(H,22,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292122
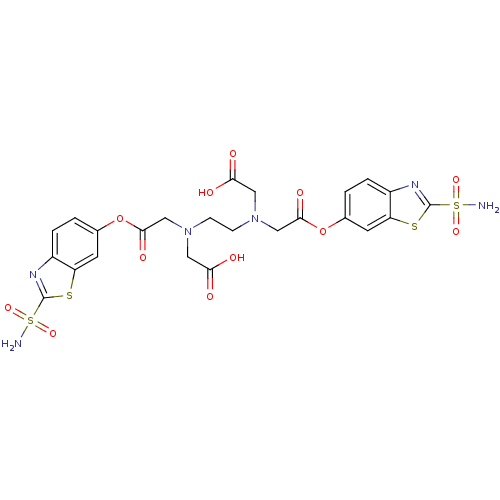
(CHEMBL34488 | [{2-[Carboxymethyl-(2-sulfamoyl-benz...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C24H24N6O12S4/c25-45(37,38)23-27-15-3-1-13(7-17(15)43-23)41-21(35)11-29(9-19(31)32)5-6-30(10-20(33)34)12-22(36)42-14-2-4-16-18(8-14)44-24(28-16)46(26,39)40/h1-4,7-8H,5-6,9-12H2,(H,31,32)(H,33,34)(H2,25,37,38)(H2,26,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50097295
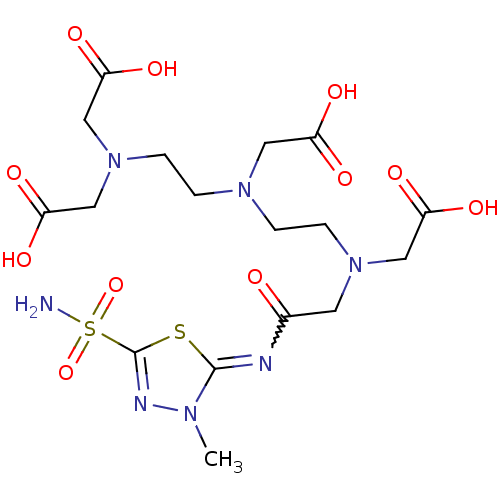
((Carboxymethyl-{2-[carboxymethyl-(2-{carboxymethyl...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)CC(O)=O)S(N)(=O)=O |w:6.7| Show InChI InChI=1S/C17H27N7O11S2/c1-21-16(36-17(20-21)37(18,34)35)19-11(25)6-23(8-13(28)29)4-2-22(7-12(26)27)3-5-24(9-14(30)31)10-15(32)33/h2-10H2,1H3,(H,26,27)(H,28,29)(H,30,31)(H,32,33)(H2,18,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292010
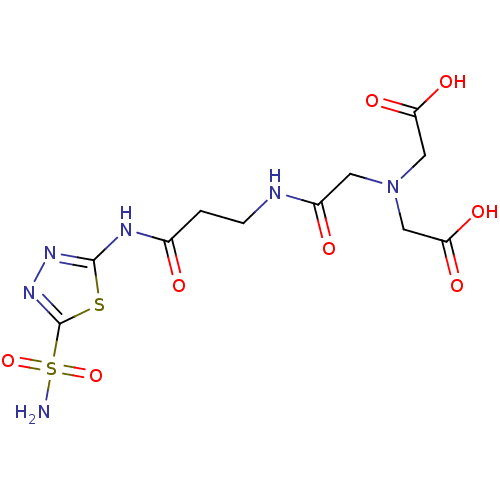
((Carboxymethyl-{[2-(5-sulfamoyl-[1,3,4]thiadiazol-...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C11H16N6O8S2/c12-27(24,25)11-16-15-10(26-11)14-6(18)1-2-13-7(19)3-17(4-8(20)21)5-9(22)23/h1-5H2,(H,13,19)(H,20,21)(H,22,23)(H2,12,24,25)(H,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292075
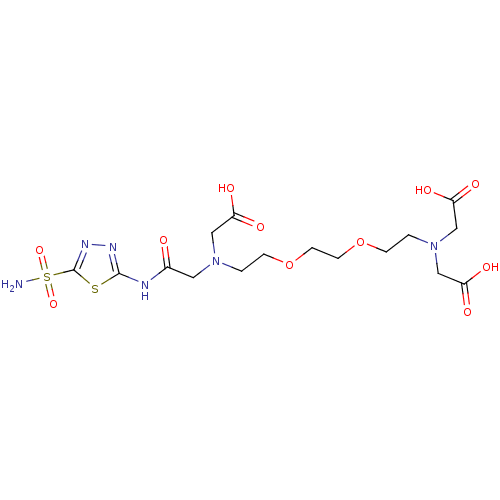
((Carboxymethyl-{2-[2-(2-{carboxymethyl-[(5-sulfamo...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C16H26N6O11S2/c17-35(30,31)16-20-19-15(34-16)18-11(23)7-21(8-12(24)25)1-3-32-5-6-33-4-2-22(9-13(26)27)10-14(28)29/h1-10H2,(H,24,25)(H,26,27)(H,28,29)(H2,17,30,31)(H,18,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292106
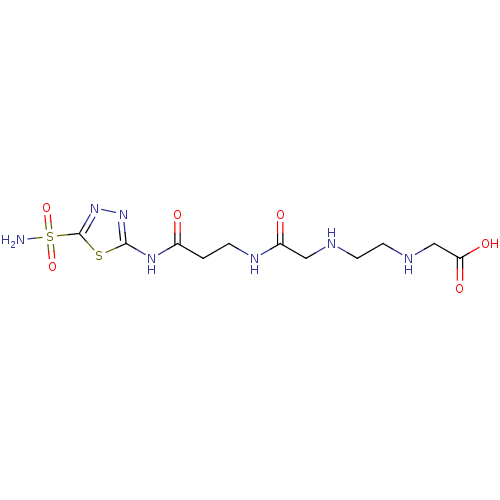
(CHEMBL33648 | [2-({[2-(5-Sulfamoyl-[1,3,4]thiadiaz...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CNCCNCC(O)=O)s1 Show InChI InChI=1S/C11H19N7O6S2/c12-26(23,24)11-18-17-10(25-11)16-7(19)1-2-15-8(20)5-13-3-4-14-6-9(21)22/h13-14H,1-6H2,(H,15,20)(H,21,22)(H2,12,23,24)(H,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292007

(CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C18H28N10O12S4/c19-43(35,36)17-25-23-15(41-17)21-11(29)7-27(9-13(31)32)1-3-39-5-6-40-4-2-28(10-14(33)34)8-12(30)22-16-24-26-18(42-16)44(20,37)38/h1-10H2,(H,31,32)(H,33,34)(H2,19,35,36)(H2,20,37,38)(H,21,23,29)(H,22,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292086
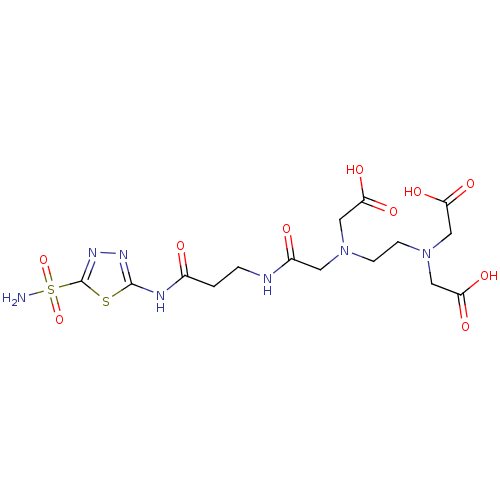
(([2-(Bis-carboxymethyl-amino)-ethyl]-{[2-(5-sulfam...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)s1 Show InChI InChI=1S/C15H23N7O10S2/c16-34(31,32)15-20-19-14(33-15)18-9(23)1-2-17-10(24)5-21(6-11(25)26)3-4-22(7-12(27)28)8-13(29)30/h1-8H2,(H,17,24)(H,25,26)(H,27,28)(H,29,30)(H2,16,31,32)(H,18,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292087
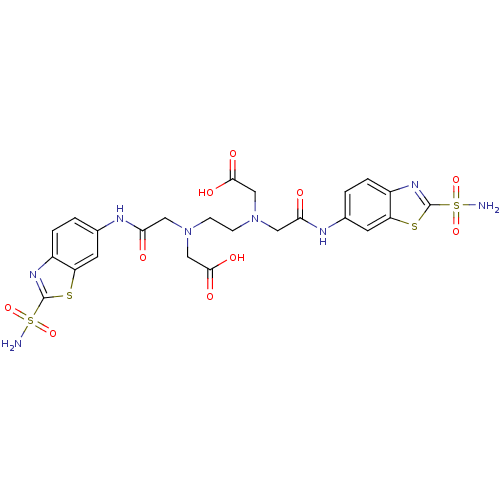
(CHEMBL34253 | {(2-{Carboxymethyl-[(2-sulfamoyl-ben...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C24H26N8O10S4/c25-45(39,40)23-29-15-3-1-13(7-17(15)43-23)27-19(33)9-31(11-21(35)36)5-6-32(12-22(37)38)10-20(34)28-14-2-4-16-18(8-14)44-24(30-16)46(26,41)42/h1-4,7-8H,5-6,9-12H2,(H,27,33)(H,28,34)(H,35,36)(H,37,38)(H2,25,39,40)(H2,26,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292045
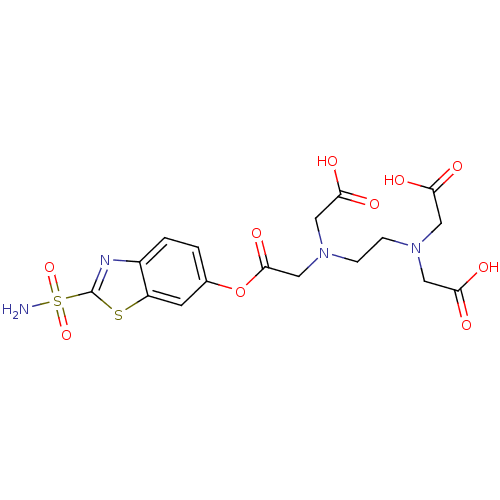
((Carboxymethyl-{2-[carboxymethyl-(2-sulfamoyl-benz...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C17H20N4O10S2/c18-33(29,30)17-19-11-2-1-10(5-12(11)32-17)31-16(28)9-21(8-15(26)27)4-3-20(6-13(22)23)7-14(24)25/h1-2,5H,3-4,6-9H2,(H,22,23)(H,24,25)(H,26,27)(H2,18,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292090
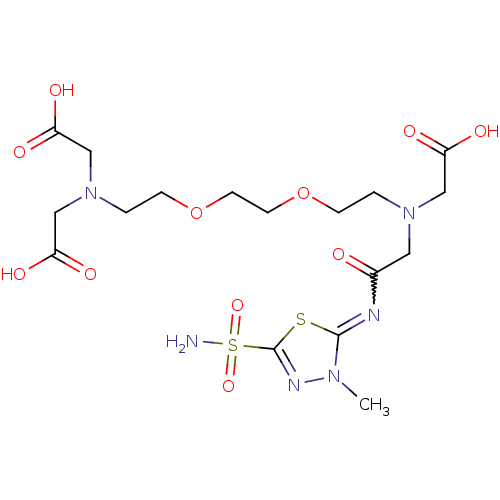
((Carboxymethyl-{2-[2-(2-{carboxymethyl-[(3-methyl-...)Show SMILES Cn1nc(sc1=NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O)S(N)(=O)=O |w:6.7| Show InChI InChI=1S/C17H28N6O11S2/c1-21-16(35-17(20-21)36(18,31)32)19-12(24)8-22(9-13(25)26)2-4-33-6-7-34-5-3-23(10-14(27)28)11-15(29)30/h2-11H2,1H3,(H,25,26)(H,27,28)(H,29,30)(H2,18,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292151
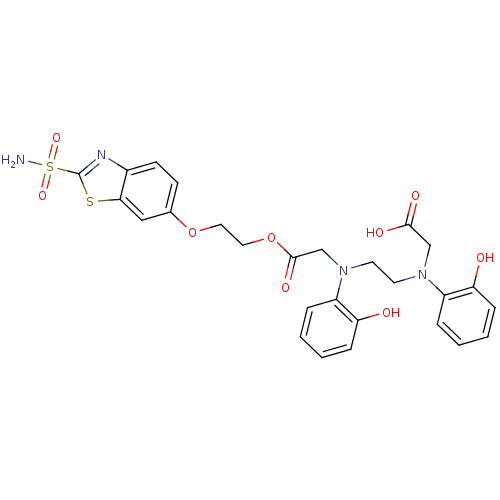
(CHEMBL32308 | [(2-Hydroxy-phenyl)-(2-{(2-hydroxy-p...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CCN(CC(O)=O)c3ccccc3O)c3ccccc3O)cc2s1 Show InChI InChI=1S/C27H28N4O9S2/c28-42(37,38)27-29-19-10-9-18(15-24(19)41-27)39-13-14-40-26(36)17-31(21-6-2-4-8-23(21)33)12-11-30(16-25(34)35)20-5-1-3-7-22(20)32/h1-10,15,32-33H,11-14,16-17H2,(H,34,35)(H2,28,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292027
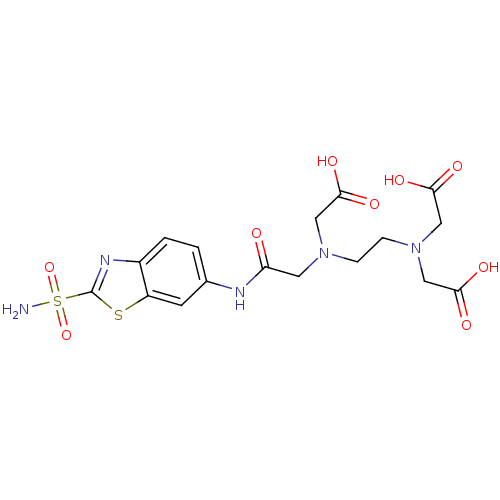
(CHEMBL34710 | [Carboxymethyl-(2-{carboxymethyl-[(2...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C17H21N5O9S2/c18-33(30,31)17-20-11-2-1-10(5-12(11)32-17)19-13(23)6-21(7-14(24)25)3-4-22(8-15(26)27)9-16(28)29/h1-2,5H,3-4,6-9H2,(H,19,23)(H,24,25)(H,26,27)(H,28,29)(H2,18,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292073
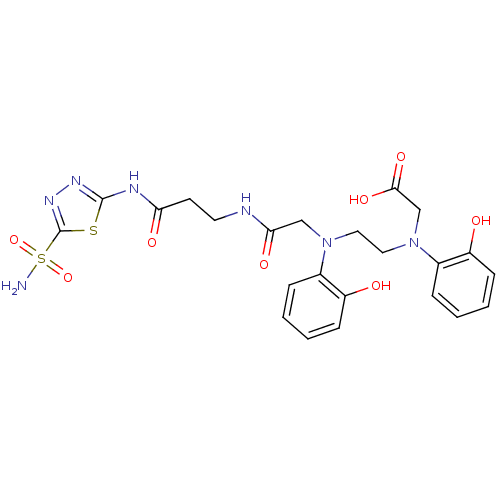
(CHEMBL35061 | {(2-Hydroxy-phenyl)-[2-((2-hydroxy-p...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CCNC(=O)CN(CCN(CC(O)=O)c2ccccc2O)c2ccccc2O)s1 Show InChI InChI=1S/C23H27N7O8S2/c24-40(37,38)23-28-27-22(39-23)26-19(33)9-10-25-20(34)13-29(15-5-1-3-7-17(15)31)11-12-30(14-21(35)36)16-6-2-4-8-18(16)32/h1-8,31-32H,9-14H2,(H,25,34)(H,35,36)(H2,24,37,38)(H,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079068
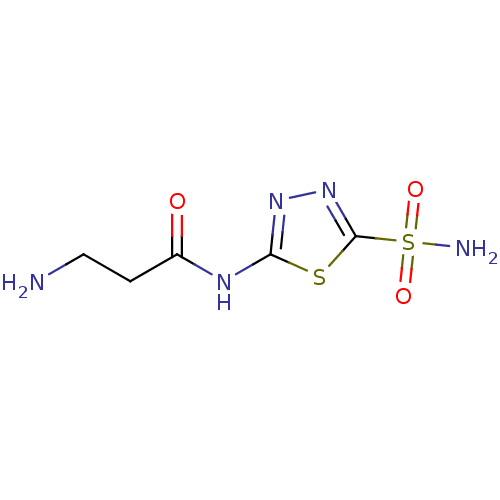
(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human Carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50292007

(CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1 Show InChI InChI=1S/C18H28N10O12S4/c19-43(35,36)17-25-23-15(41-17)21-11(29)7-27(9-13(31)32)1-3-39-5-6-40-4-2-28(10-14(33)34)8-12(30)22-16-24-26-18(42-16)44(20,37)38/h1-10H2,(H,31,32)(H,33,34)(H2,19,35,36)(H2,20,37,38)(H,21,23,29)(H,22,24,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50097307

(CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Oc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H31N7O14S4/c29-52(44,45)27-31-18-3-1-16(9-20(18)50-27)48-25(42)14-34(12-23(38)39)7-5-33(11-22(36)37)6-8-35(13-24(40)41)15-26(43)49-17-2-4-19-21(10-17)51-28(32-19)53(30,46)47/h1-4,9-10H,5-8,11-15H2,(H,36,37)(H,38,39)(H,40,41)(H2,29,44,45)(H2,30,46,47) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50292100

(CHEMBL284354 | {{2-[2-(2-{Carboxymethyl-[(2-sulfam...)Show SMILES NS(=O)(=O)c1nc2ccc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc3ccc4nc(sc4c3)S(N)(=O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C28H34N8O12S4/c29-51(43,44)27-33-19-3-1-17(11-21(19)49-27)31-23(37)13-35(15-25(39)40)5-7-47-9-10-48-8-6-36(16-26(41)42)14-24(38)32-18-2-4-20-22(12-18)50-28(34-20)52(30,45)46/h1-4,11-12H,5-10,13-16H2,(H,31,37)(H,32,38)(H,39,40)(H,41,42)(H2,29,43,44)(H2,30,45,46) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292039
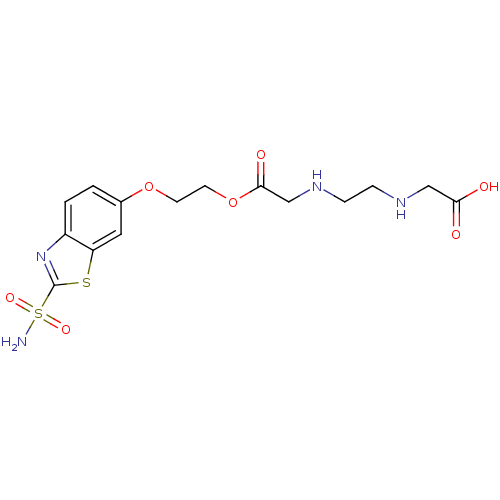
((2-{[2-(2-Sulfamoyl-benzothiazol-6-yloxy)-ethoxyca...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CNCCNCC(O)=O)cc2s1 Show InChI InChI=1S/C15H20N4O7S2/c16-28(23,24)15-19-11-2-1-10(7-12(11)27-15)25-5-6-26-14(22)9-18-4-3-17-8-13(20)21/h1-2,7,17-18H,3-6,8-9H2,(H,20,21)(H2,16,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292116
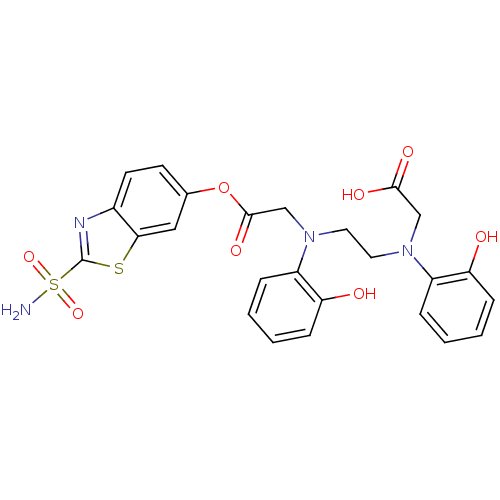
(((2-Hydroxy-phenyl)-{2-[(2-hydroxy-phenyl)-(2-sulf...)Show SMILES NS(=O)(=O)c1nc2ccc(OC(=O)CN(CCN(CC(O)=O)c3ccccc3O)c3ccccc3O)cc2s1 Show InChI InChI=1S/C25H24N4O8S2/c26-39(35,36)25-27-17-10-9-16(13-22(17)38-25)37-24(34)15-29(19-6-2-4-8-21(19)31)12-11-28(14-23(32)33)18-5-1-3-7-20(18)30/h1-10,13,30-31H,11-12,14-15H2,(H,32,33)(H2,26,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50292043
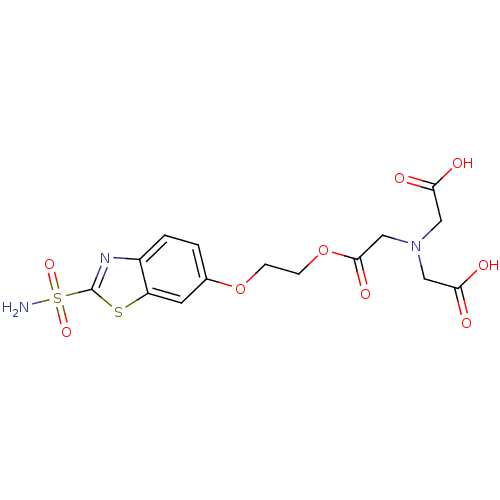
(CHEMBL289401 | {Carboxymethyl-[2-(2-sulfamoyl-benz...)Show SMILES NS(=O)(=O)c1nc2ccc(OCCOC(=O)CN(CC(O)=O)CC(O)=O)cc2s1 Show InChI InChI=1S/C15H17N3O9S2/c16-29(24,25)15-17-10-2-1-9(5-11(10)28-15)26-3-4-27-14(23)8-18(6-12(19)20)7-13(21)22/h1-2,5H,3-4,6-8H2,(H,19,20)(H,21,22)(H2,16,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human carbonic anhydrase II |
J Med Chem 45: 1466-76 (2002)
BindingDB Entry DOI: 10.7270/Q2N29XN9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data