

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50111438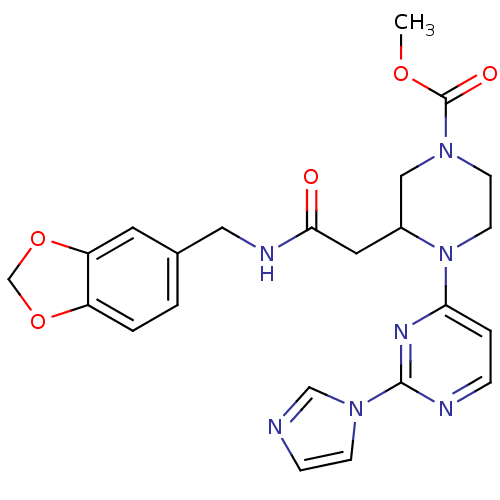 (3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding against the partially purified human Inducible nitric oxide synthase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50111438 (3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against the partially purified human Inducible nitric oxide synthase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50111448 (CHEMBL440473 | N-Benzo[1,3]dioxol-5-ylmethyl-2-[1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against the partially purified human Inducible nitric oxide synthase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of chemotactic protein to CCR5 | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50078347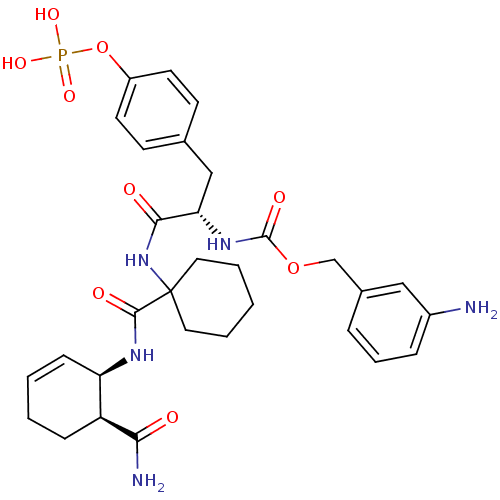 (CHEMBL307890 | [(S)-1-[1-((1R,6S)-6-Carbamoyl-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | MMDB PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Grb2-SH2 domain binding to phospho-EGF receptor intracellular C-terminal domain | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glutathione S-transferase A2 (Homo sapiens (Human)) | BDBM50111440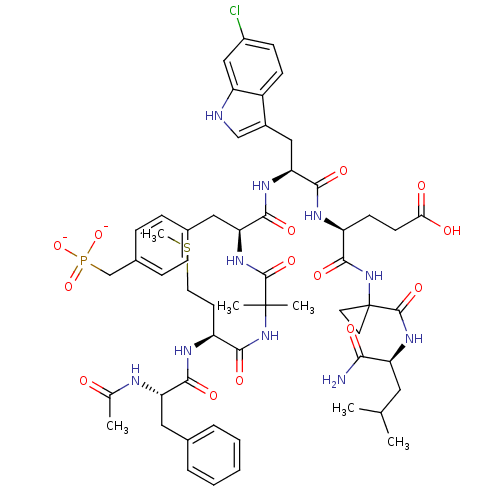 (4-(1-carbamoyl-4-methylpentanamide-2-yl-cyclopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of p53 binding to Glutathione S-transferase 2 (hdm2-GST) | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of chemotactic protein to CCR2b | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50111441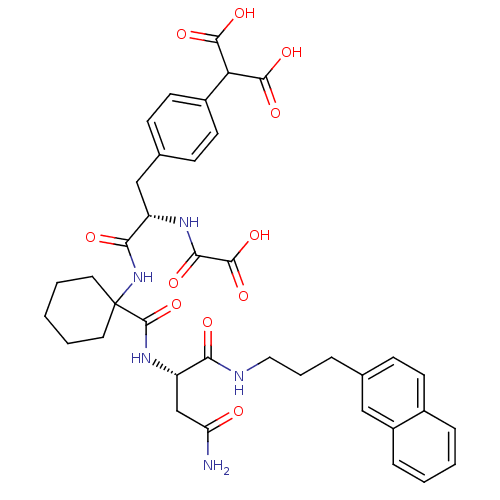 (CHEMBL288116 | [1-[1-(6-Carbamoyl-cyclohex-2-enylc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Growth factor receptor bound protein 2 SH2-domain binding | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C5a anaphylatoxin chemotactic receptor 1 (Homo sapiens (Human)) | BDBM50111445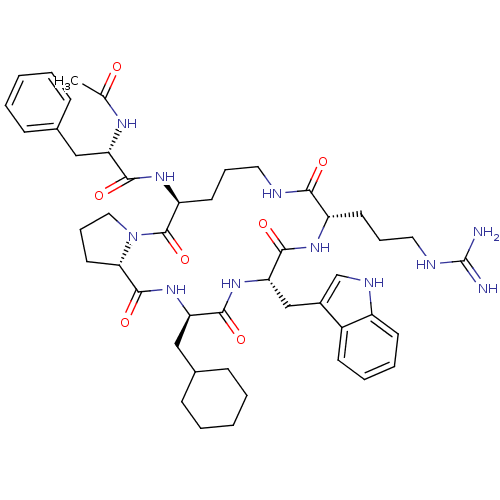 ((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity against the C5a receptor | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50111439 (CHEMBL42853 | {4-[2-Acetylamino-2-(3-carbamoyl-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50111443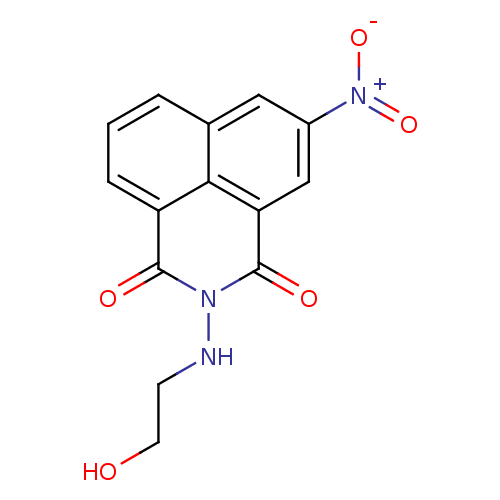 (2-(2-Hydroxy-ethylamino)-5-nitro-benzo[de]isoquino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of NGF-stimulated Trk A | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 16 (Homo sapiens (Human)) | BDBM50111443 (2-(2-Hydroxy-ethylamino)-5-nitro-benzo[de]isoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Competitive binding against Nerve growth factor to p75-NGF receptor in PC12 cells | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50111437 (4-[2-Acetylamino-2-(3-carbamoyl-2-cyclohexylmethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of binding to Src SH2 domain | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50111443 (2-(2-Hydroxy-ethylamino)-5-nitro-benzo[de]isoquino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of NGF-stimulated Trk A | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50111444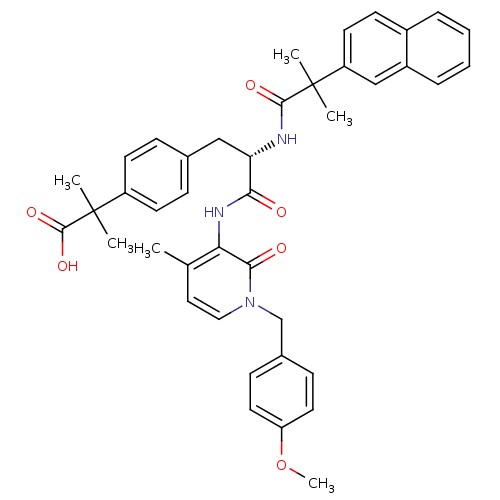 (2-{4-[2-[1-(4-Methoxy-benzyl)-4-methyl-2-oxo-1,2-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of calcium release in Jurkat cells after T-cell receptor cross linking antibody treatment | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50111444 (2-{4-[2-[1-(4-Methoxy-benzyl)-4-methyl-2-oxo-1,2-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for p56 lck kinase | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50111444 (2-{4-[2-[1-(4-Methoxy-benzyl)-4-methyl-2-oxo-1,2-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of p56 Lck SH2 domain binding to pYEEI | J Med Chem 45: 1543-58 (2002) BindingDB Entry DOI: 10.7270/Q2CN74ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||