

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50367879 (LISINOPRIL) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130714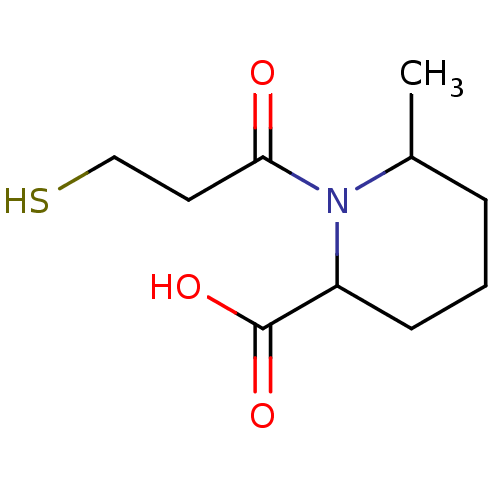 (1-(3-Mercapto-propionyl)-6-methyl-piperidine-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidney | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130714 (1-(3-Mercapto-propionyl)-6-methyl-piperidine-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50130714 (1-(3-Mercapto-propionyl)-6-methyl-piperidine-2-car...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130716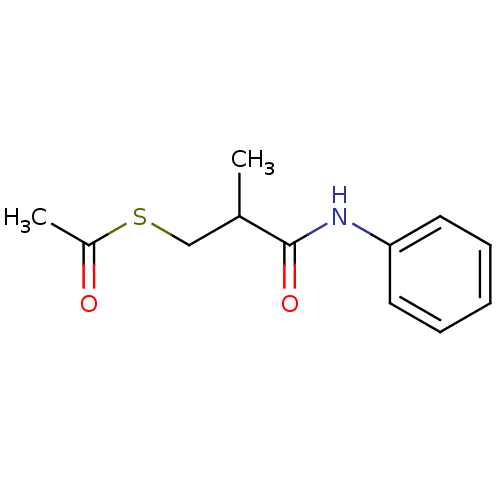 (CHEMBL322069 | Thioacetic acid S-(2-phenylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidney | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50130716 (CHEMBL322069 | Thioacetic acid S-(2-phenylcarbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130716 (CHEMBL322069 | Thioacetic acid S-(2-phenylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50130713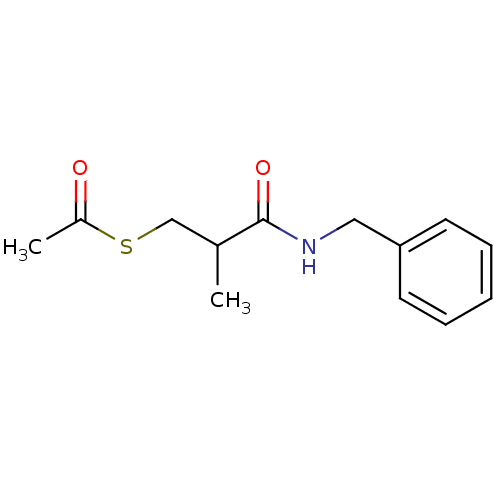 (CHEMBL326801 | Thioacetic acid S-(2-benzylcarbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50130716 (CHEMBL322069 | Thioacetic acid S-(2-phenylcarbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50130713 (CHEMBL326801 | Thioacetic acid S-(2-benzylcarbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130715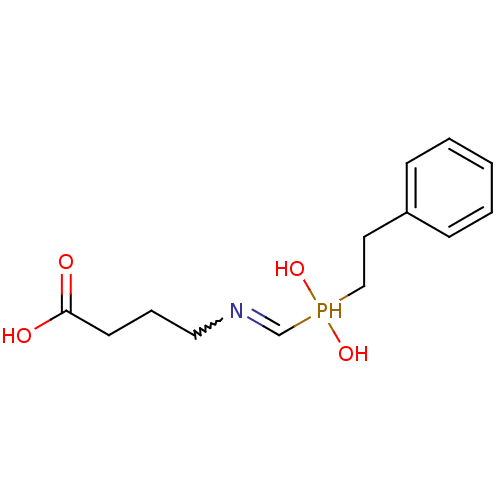 (4-[(Hydroxy-phenethyl-phosphinoylmethyl)-amino]-bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50130715 (4-[(Hydroxy-phenethyl-phosphinoylmethyl)-amino]-bu...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130713 (CHEMBL326801 | Thioacetic acid S-(2-benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serum | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50130716 (CHEMBL322069 | Thioacetic acid S-(2-phenylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50130713 (CHEMBL326801 | Thioacetic acid S-(2-benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50130713 (CHEMBL326801 | Thioacetic acid S-(2-benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme (ACE) from bovine kidney | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50130715 (4-[(Hydroxy-phenethyl-phosphinoylmethyl)-amino]-bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50130714 (1-(3-Mercapto-propionyl)-6-methyl-piperidine-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomedical Chemistry of Russian Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP) from rat cortex brain membrane | J Med Chem 46: 3326-32 (2003) Article DOI: 10.1021/jm021089h BindingDB Entry DOI: 10.7270/Q2K07512 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||