 Found 76 hits Enz. Inhib. hit(s) with all data for entry = 50037270
Found 76 hits Enz. Inhib. hit(s) with all data for entry = 50037270 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22920

(1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...)Show SMILES Cn1c(CCC(=O)Nc2ccc3ccn(CC[C@H](CO)n4cnc(c4)C(N)=O)c3c2)nc2ccccc12 |r| Show InChI InChI=1S/C27H29N7O3/c1-32-23-5-3-2-4-21(23)31-25(32)8-9-26(36)30-19-7-6-18-10-12-33(24(18)14-19)13-11-20(16-35)34-15-22(27(28)37)29-17-34/h2-7,10,12,14-15,17,20,35H,8-9,11,13,16H2,1H3,(H2,28,37)(H,30,36)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine deaminase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50149217

(5-{[5-((R)-1-Carboxy-2-oxo-ethylcarbamoyl)-pyrimid...)Show SMILES OC(=O)[C@H](NC(=O)c1cnc(CNS(=O)(=O)c2ccc(O)c(c2)C(O)=O)nc1)C=O Show InChI InChI=1S/C16H14N4O9S/c21-7-11(16(26)27)20-14(23)8-4-17-13(18-5-8)6-19-30(28,29)9-1-2-12(22)10(3-9)15(24)25/h1-5,7,11,19,22H,6H2,(H,20,23)(H,24,25)(H,26,27)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Caspase-3 |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50149232
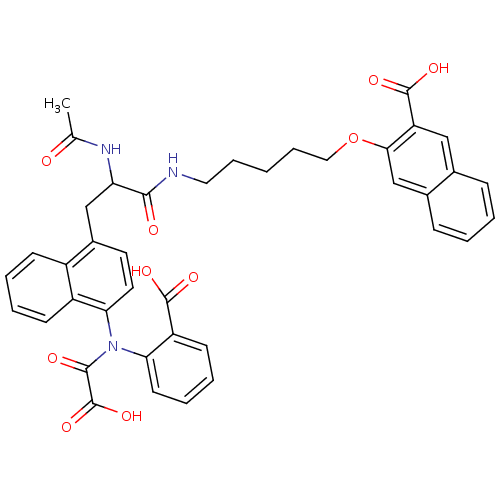
(3-(5-(2-acetamido-3-(4-(carboxy-N-(2-carboxyphenyl...)Show SMILES CC(=O)NC(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147086
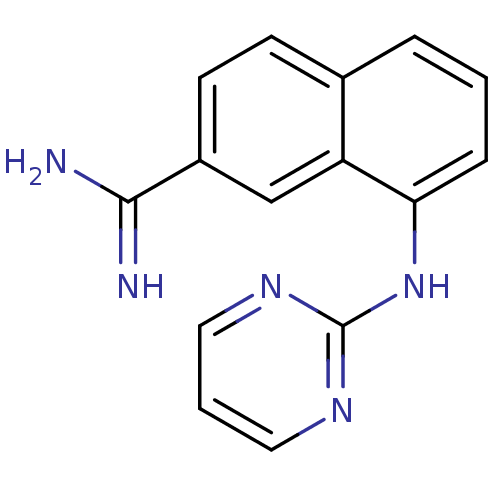
(8-(PYRIMIDIN-2-YLAMINO)NAPHTHALENE-2-CARBOXIMIDAMI...)Show InChI InChI=1S/C15H13N5/c16-14(17)11-6-5-10-3-1-4-13(12(10)9-11)20-15-18-7-2-8-19-15/h1-9H,(H3,16,17)(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131555

(2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C33H35N3O11/c1-3-21-17-20(13-14-24(21)36(30(40)33(45)46)25-10-5-4-9-22(25)31(41)42)18-23(35-19(2)37)29(39)34-15-6-7-16-47-27-12-8-11-26(38)28(27)32(43)44/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,34,39)(H,35,37)(H,41,42)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Lactobacillus casei) | BDBM50149206

((S)-2-{4-[(R)-2-(2-Carboxy-ethylcarbamoyl)-pyrroli...)Show SMILES OC(=O)CCNC(=O)[C@H]1CCCN1S(=O)(=O)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H25N3O10S/c24-16(25)8-7-14(20(30)31)22-18(28)12-3-5-13(6-4-12)34(32,33)23-11-1-2-15(23)19(29)21-10-9-17(26)27/h3-6,14-15H,1-2,7-11H2,(H,21,29)(H,22,28)(H,24,25)(H,26,27)(H,30,31)/t14-,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards thymidylate synthase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50149214

(CHEMBL117368 | N*8*-Pyrimidin-2-yl-quinoline-2,8-d...)Show InChI InChI=1S/C13H11N5/c14-11-6-5-9-3-1-4-10(12(9)18-11)17-13-15-7-2-8-16-13/h1-8H,(H2,14,18)(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22919

(1-[1-hydroxy-4-(naphthalen-1-yl)butan-2-yl]-1H-imi...)Show InChI InChI=1S/C18H19N3O2/c19-18(23)17-10-21(12-20-17)15(11-22)9-8-14-6-3-5-13-4-1-2-7-16(13)14/h1-7,10,12,15,22H,8-9,11H2,(H2,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine deaminase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50149239
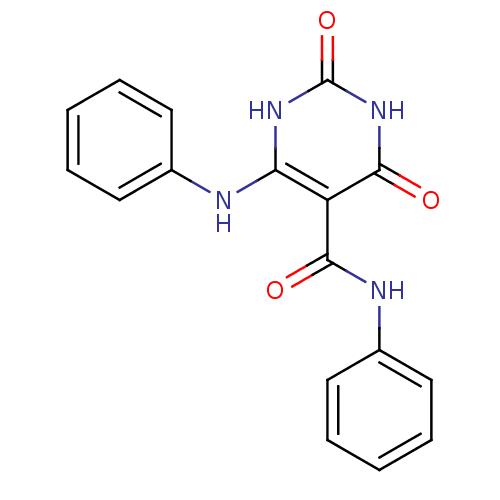
(2,4-Dioxo-6-phenylamino-1,2,3,4-tetrahydro-pyrimid...)Show SMILES O=C(Nc1ccccc1)c1c(Nc2ccccc2)[nH]c(=O)[nH]c1=O Show InChI InChI=1S/C17H14N4O3/c22-15(19-12-9-5-2-6-10-12)13-14(20-17(24)21-16(13)23)18-11-7-3-1-4-8-11/h1-10H,(H,19,22)(H3,18,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Affinity for c-Jun N-terminal kinase 3 |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13953
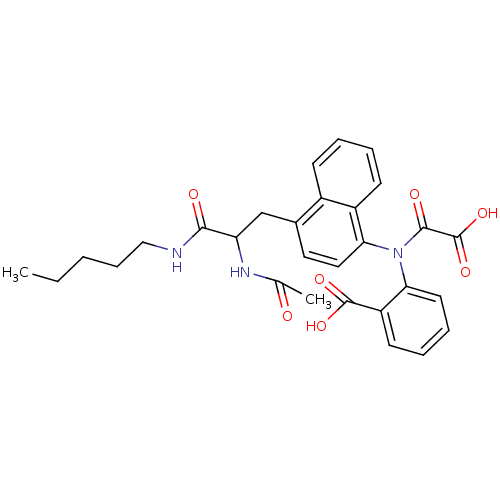
(1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...)Show SMILES CCCCCNC(=O)C(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)NC(C)=O Show InChI InChI=1S/C29H31N3O7/c1-3-4-9-16-30-26(34)23(31-18(2)33)17-19-14-15-25(21-11-6-5-10-20(19)21)32(27(35)29(38)39)24-13-8-7-12-22(24)28(36)37/h5-8,10-15,23H,3-4,9,16-17H2,1-2H3,(H,30,34)(H,31,33)(H,36,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50149224
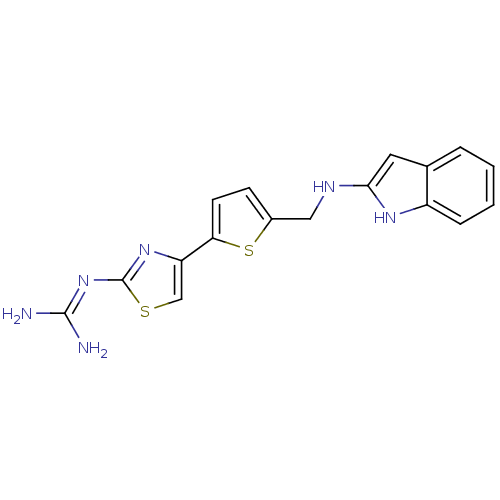
(CHEMBL118106 | N-(4-{5-[(1H-Indol-2-ylamino)-methy...)Show SMILES NC(N)=Nc1nc(cs1)-c1ccc(CNc2cc3ccccc3[nH]2)s1 |(14.54,.89,;13.04,1.21,;12,.06,;12.56,2.68,;11.06,3,;9.92,1.96,;8.59,2.75,;8.9,4.24,;10.44,4.4,;7.17,2.12,;5.84,2.89,;4.7,1.86,;5.33,.46,;4.56,-.87,;3.02,-.87,;2.25,-2.22,;.71,-2.36,;.38,-3.88,;-.95,-4.65,;-.95,-6.19,;.38,-6.96,;1.73,-6.19,;1.73,-4.65,;2.88,-3.62,;6.84,.61,)| Show InChI InChI=1S/C17H16N6S2/c18-16(19)23-17-22-13(9-24-17)14-6-5-11(25-14)8-20-15-7-10-3-1-2-4-12(10)21-15/h1-7,9,20-21H,8H2,(H4,18,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards adenosine deaminase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13971

(1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...)Show SMILES CCCCCNC(=O)C(Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c(CC)c1)NC(C)=O Show InChI InChI=1S/C27H33N3O7/c1-4-6-9-14-28-24(32)21(29-17(3)31)16-18-12-13-22(19(5-2)15-18)30(25(33)27(36)37)23-11-8-7-10-20(23)26(34)35/h7-8,10-13,15,21H,4-6,9,14,16H2,1-3H3,(H,28,32)(H,29,31)(H,34,35)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Caspase-3
(Homo sapiens (Human)) | BDBM50149205
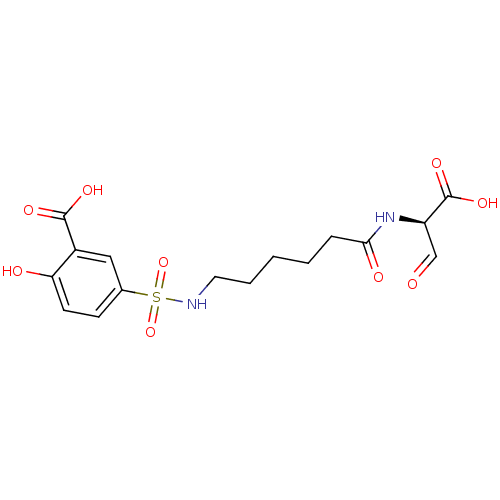
(5-[5-((R)-1-Carboxy-2-oxo-ethylcarbamoyl)-pentylsu...)Show SMILES OC(=O)[C@H](NC(=O)CCCCCNS(=O)(=O)c1ccc(O)c(c1)C(O)=O)C=O Show InChI InChI=1S/C16H20N2O9S/c19-9-12(16(24)25)18-14(21)4-2-1-3-7-17-28(26,27)10-5-6-13(20)11(8-10)15(22)23/h5-6,8-9,12,17,20H,1-4,7H2,(H,18,21)(H,22,23)(H,24,25)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Caspase-3 |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13990
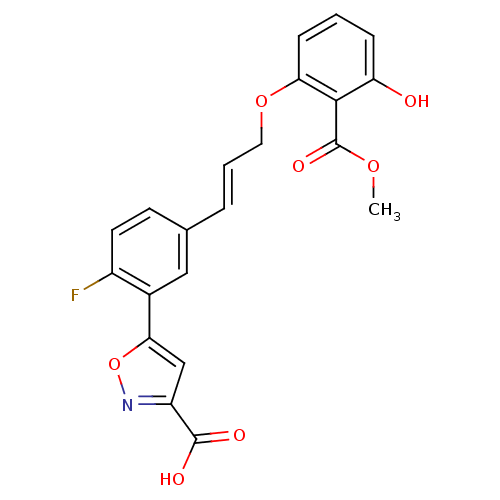
(5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1ccc(F)c(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H16FNO7/c1-28-21(27)19-16(24)5-2-6-17(19)29-9-3-4-12-7-8-14(22)13(10-12)18-11-15(20(25)26)23-30-18/h2-8,10-11,24H,9H2,1H3,(H,25,26)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50149200

(2-Pyridin-4-yl-thiazole-4-carboxylic acid (3-trifl...)Show InChI InChI=1S/C16H10F3N3OS/c17-16(18,19)11-2-1-3-12(8-11)21-14(23)13-9-24-15(22-13)10-4-6-20-7-5-10/h1-9H,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Affinity for c-Jun N-terminal kinase 3 |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50149202

(4-[[GLUTAMIC ACID]-CARBONYL]-BENZENE-SULFONYL-D-PR...)Show SMILES OC(=O)CC[C@H](NC(=O)c1ccc(cc1)S(=O)(=O)N1CCC[C@@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C17H20N2O9S/c20-14(21)8-7-12(16(23)24)18-15(22)10-3-5-11(6-4-10)29(27,28)19-9-1-2-13(19)17(25)26/h3-6,12-13H,1-2,7-9H2,(H,18,22)(H,20,21)(H,23,24)(H,25,26)/t12-,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards thymidylate synthase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13952
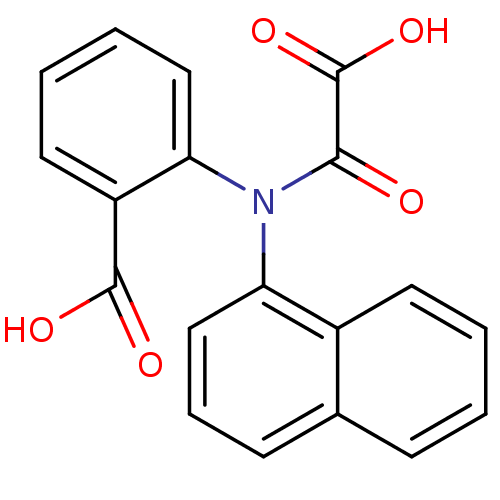
(2-(Naphthalen-1-yloxalylamino)benzoic Acid | 2-(na...)Show InChI InChI=1S/C19H13NO5/c21-17(19(24)25)20(16-10-4-3-9-14(16)18(22)23)15-11-5-7-12-6-1-2-8-13(12)15/h1-11H,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50149208

(2-Amino-quinolin-8-ol | CHEMBL119647)Show InChI InChI=1S/C9H8N2O/c10-8-5-4-6-2-1-3-7(12)9(6)11-8/h1-5,12H,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Urokinase-type plasminogen activator |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50133279
(5-(3-Butyrylamino-phenyl)-isoxazole-3-carboxylic a...)Show InChI InChI=1S/C14H14N2O4/c1-2-4-13(17)15-10-6-3-5-9(7-10)12-8-11(14(18)19)16-20-12/h3,5-8H,2,4H2,1H3,(H,15,17)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13951
(2-[(2,3-Dimethylphenyl)oxalylamino]benzoic acid | ...)Show InChI InChI=1S/C17H15NO5/c1-10-6-5-9-13(11(10)2)18(15(19)17(22)23)14-8-4-3-7-12(14)16(20)21/h3-9H,1-2H3,(H,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Protein-tyrosine phosphatase 1B |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Caspase-3
(Homo sapiens (Human)) | BDBM50149213
(2-Hydroxy-5-methylsulfamoyl-benzoic acid | CHEMBL1...)Show InChI InChI=1S/C8H9NO5S/c1-9-15(13,14)5-2-3-7(10)6(4-5)8(11)12/h2-4,9-10H,1H3,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Caspase-3 |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50149243
((R)-1-(Toluene-4-sulfonyl)-pyrrolidine-2-carboxyli...)Show InChI InChI=1S/C12H15NO4S/c1-9-4-6-10(7-5-9)18(16,17)13-8-2-3-11(13)12(14)15/h4-7,11H,2-3,8H2,1H3,(H,14,15)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Tested for binding affinity towards thymidylate synthase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50149212

(CHEMBL117521 | N-(1,2,3,4,8a,10a-Hexahydro-acridin...)Show SMILES C(CCCNC1=C2CCCCC2=NC2C=CCC=C12)CCCNc1c2CCCCc2nc2ccccc12 |c:5,12,15,t:18| Show InChI InChI=1S/C33H42N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,8,10,14,16,18,20,30,35H,1-3,5-7,9,11-13,15,17,19,21-23H2,(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase in rat brain |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of influenza A viral enzyme neuraminidase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50094703
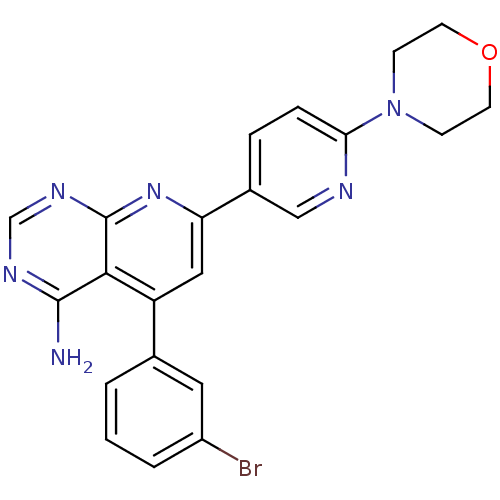
(5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-y...)Show SMILES Nc1ncnc2nc(cc(-c3cccc(Br)c3)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C22H19BrN6O/c23-16-3-1-2-14(10-16)17-11-18(28-22-20(17)21(24)26-13-27-22)15-4-5-19(25-12-15)29-6-8-30-9-7-29/h1-5,10-13H,6-9H2,(H2,24,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine kinase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50149207

(2N-(2-{2-[2-(5-chloro-1H-2-indolylcarboxamido)etho...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCCOCCOCCNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C24H24Cl2N4O4/c25-17-1-3-19-15(11-17)13-21(29-19)23(31)27-5-7-33-9-10-34-8-6-28-24(32)22-14-16-12-18(26)2-4-20(16)30-22/h1-4,11-14,29-30H,5-10H2,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50094704
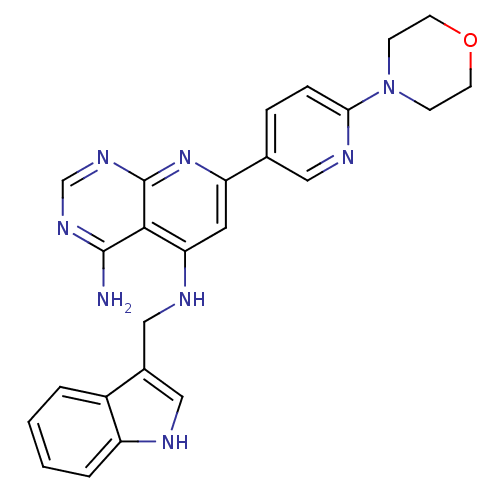
(CHEMBL116872 | N*5*-(1H-Indol-3-ylmethyl)-7-(6-mor...)Show SMILES Nc1ncnc2nc(cc(NCc3c[nH]c4ccccc34)c12)-c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C25H24N8O/c26-24-23-21(28-14-17-13-27-19-4-2-1-3-18(17)19)11-20(32-25(23)31-15-30-24)16-5-6-22(29-12-16)33-7-9-34-10-8-33/h1-6,11-13,15,27H,7-10,14H2,(H3,26,28,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against adenosine kinase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149219
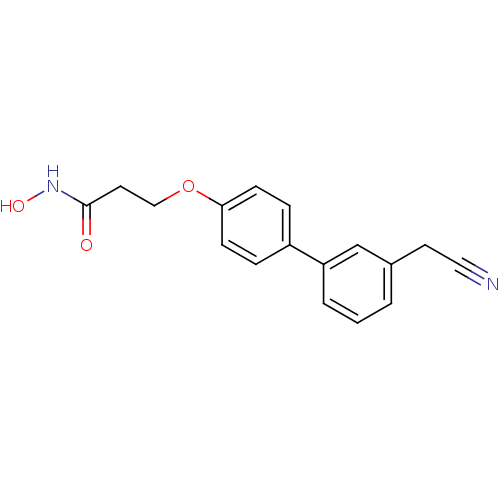
(3-(3'-Cyanomethyl-biphenyl-4-yloxy)-N-hydroxy-prop...)Show InChI InChI=1S/C17H16N2O3/c18-10-8-13-2-1-3-15(12-13)14-4-6-16(7-5-14)22-11-9-17(20)19-21/h1-7,12,21H,8-9,11H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50149235

(2-[Benzyl-(4-methoxy-benzenesulfonyl)-amino]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(C)cc(C)cc1C(=O)NO Show InChI InChI=1S/C23H24N2O5S/c1-16-13-17(2)22(21(14-16)23(26)24-27)25(15-18-7-5-4-6-8-18)31(28,29)20-11-9-19(30-3)10-12-20/h4-14,27H,15H2,1-3H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-13 (MMP-13) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50149216
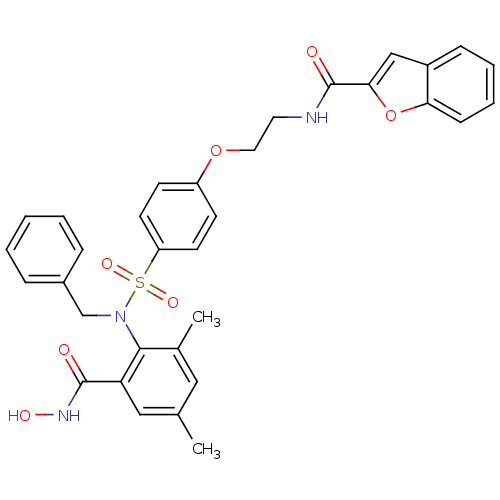
(Benzofuran-2-carboxylic acid (2-{4-[benzyl-(2-hydr...)Show SMILES Cc1cc(C)c(N(Cc2ccccc2)S(=O)(=O)c2ccc(OCCNC(=O)c3cc4ccccc4o3)cc2)c(c1)C(=O)NO Show InChI InChI=1S/C33H31N3O7S/c1-22-18-23(2)31(28(19-22)32(37)35-39)36(21-24-8-4-3-5-9-24)44(40,41)27-14-12-26(13-15-27)42-17-16-34-33(38)30-20-25-10-6-7-11-29(25)43-30/h3-15,18-20,39H,16-17,21H2,1-2H3,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-13 (MMP-13) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50098760
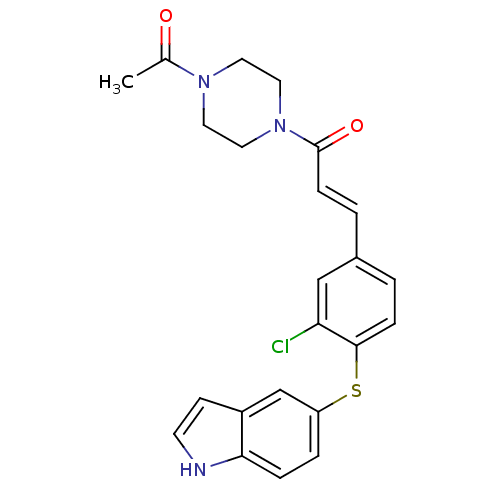
((E)-1-(4-Acetyl-piperazin-1-yl)-3-[3-chloro-4-(1H-...)Show SMILES CC(=O)N1CCN(CC1)C(=O)\C=C\c1ccc(Sc2ccc3[nH]ccc3c2)c(Cl)c1 Show InChI InChI=1S/C23H22ClN3O2S/c1-16(28)26-10-12-27(13-11-26)23(29)7-3-17-2-6-22(20(24)14-17)30-19-4-5-21-18(15-19)8-9-25-21/h2-9,14-15,25H,10-13H2,1H3/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Leukocyte function associated antigen 1 (LFA-1) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50096645
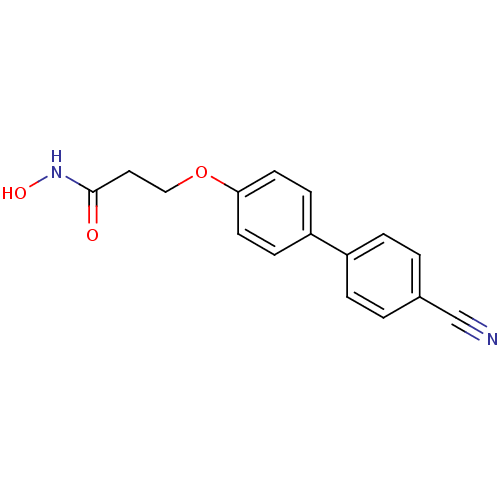
(3-(4'-Cyano-biphenyl-4-yloxy)-N-hydroxy-propionami...)Show InChI InChI=1S/C16H14N2O3/c17-11-12-1-3-13(4-2-12)14-5-7-15(8-6-14)21-10-9-16(19)18-20/h1-8,20H,9-10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7684
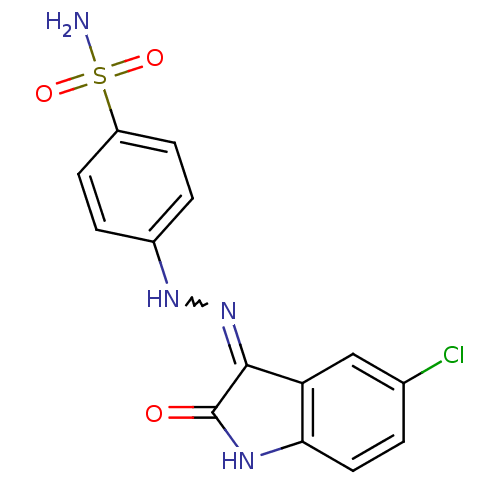
(4-[2-(5-Chloro-2-oxo-1,2-dihydro-3H-indol-3-yliden...)Show SMILES NS(=O)(=O)c1ccc(NN=C2C(=O)Nc3ccc(Cl)cc23)cc1 |w:9.8| Show InChI InChI=1S/C14H11ClN4O3S/c15-8-1-6-12-11(7-8)13(14(20)17-12)19-18-9-2-4-10(5-3-9)23(16,21)22/h1-7,18H,(H2,16,21,22)(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-Dependent Kinase 2 |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50098770

((E)-1-(4-Acetyl-piperazin-1-yl)-3-[3-chloro-4-(2,3...)Show SMILES CC(=O)N1CCN(CC1)C(=O)\C=C\c1ccc(Sc2ccc3OCCOc3c2)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-16(27)25-8-10-26(11-9-25)23(28)7-3-17-2-6-22(19(24)14-17)31-18-4-5-20-21(15-18)30-13-12-29-20/h2-7,14-15H,8-13H2,1H3/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Leukocyte function associated antigen 1 (LFA-1) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Adenylosuccinate synthetase isozyme 2
(Homo sapiens (Human)) | BDBM50149229

(5-((1R,5S,7R,8S,9R)-8,9-Dihydroxy-2,4-dioxo-7-phos...)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)O[C@@]2(NC(=O)N(CCC[C@H](N(O)C=O)C(O)=O)C2=O)[C@@H]1O Show InChI InChI=1S/C13H20N3O13P/c17-5-16(24)6(10(20)21)2-1-3-15-11(22)13(14-12(15)23)9(19)8(18)7(29-13)4-28-30(25,26)27/h5-9,18-19,24H,1-4H2,(H,14,23)(H,20,21)(H2,25,26,27)/t6-,7+,8+,9+,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Adenylosuccinate synthetase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50149226

((E)-1-(4-Acetyl-piperazin-1-yl)-3-[4-(2-isopropyl-...)Show SMILES CC(C)c1ccccc1SC1CC=C(\C=C\C(=O)N2CCN(CC2)C(C)=O)C=C1[N+]([O-])=O |c:29,t:13| Show InChI InChI=1S/C24H29N3O4S/c1-17(2)20-6-4-5-7-22(20)32-23-10-8-19(16-21(23)27(30)31)9-11-24(29)26-14-12-25(13-15-26)18(3)28/h4-9,11,16-17,23H,10,12-15H2,1-3H3/b11-9+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Leukocyte function associated antigen 1 (LFA-1) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149245
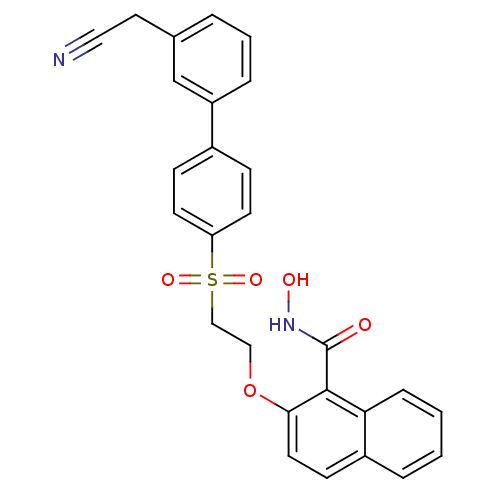
(2-[2-(3'-Cyanomethyl-biphenyl-4-sulfonyl)-ethoxy]-...)Show SMILES ONC(=O)c1c(OCCS(=O)(=O)c2ccc(cc2)-c2cccc(CC#N)c2)ccc2ccccc12 Show InChI InChI=1S/C27H22N2O5S/c28-15-14-19-4-3-6-22(18-19)20-8-11-23(12-9-20)35(32,33)17-16-34-25-13-10-21-5-1-2-7-24(21)26(25)27(30)29-31/h1-13,18,31H,14,16-17H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50149222
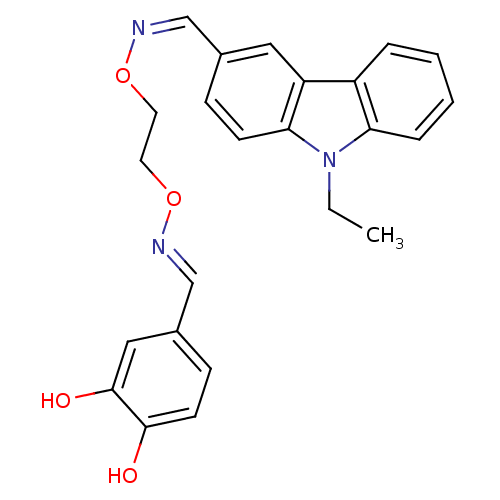
(9-Ethyl-9H-carbazole-3-carbaldehyde O-{2-[1-(3,4-d...)Show SMILES CCn1c2ccccc2c2cc(\C=N/OCCO\N=C\c3ccc(O)c(O)c3)ccc12 Show InChI InChI=1S/C24H23N3O4/c1-2-27-21-6-4-3-5-19(21)20-13-17(7-9-22(20)27)15-25-30-11-12-31-26-16-18-8-10-23(28)24(29)14-18/h3-10,13-16,28-29H,2,11-12H2,1H3/b25-15-,26-16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of c-Src tyrosine kinase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50149235

(2-[Benzyl-(4-methoxy-benzenesulfonyl)-amino]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(C)cc(C)cc1C(=O)NO Show InChI InChI=1S/C23H24N2O5S/c1-16-13-17(2)22(21(14-16)23(26)24-27)25(15-18-7-5-4-6-8-18)31(28,29)20-11-9-19(30-3)10-12-20/h4-14,27H,15H2,1-3H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-1 (MMP-1) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5234

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-ylamino...)Show SMILES CCC(CC)N[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H25N3O3/c1-4-10(5-2)17-12-7-9(14(19)20)6-11(15)13(12)16-8(3)18/h7,10-13,17H,4-6,15H2,1-3H3,(H,16,18)(H,19,20)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of influenza A viral enzyme neuraminidase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50065965
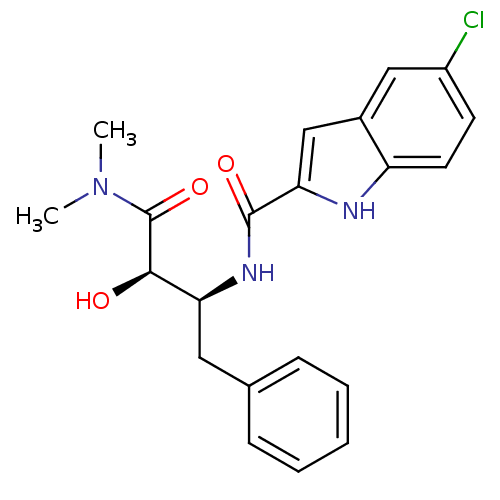
(5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...)Show SMILES CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H22ClN3O3/c1-25(2)21(28)19(26)17(10-13-6-4-3-5-7-13)24-20(27)18-12-14-11-15(22)8-9-16(14)23-18/h3-9,11-12,17,19,23,26H,10H2,1-2H3,(H,24,27)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149230

(3-(Biphenyl-4-yloxy)-N-hydroxy-propionamide | CHEM...)Show InChI InChI=1S/C15H15NO3/c17-15(16-18)10-11-19-14-8-6-13(7-9-14)12-4-2-1-3-5-12/h1-9,18H,10-11H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149220

(2-[2-(4'-Cyano-biphenyl-4-yloxy)-ethoxy]-naphthale...)Show SMILES CONC(=O)c1c(OCCOc2ccc(cc2)-c2ccc(cc2)C#N)ccc2ccccc12 Show InChI InChI=1S/C27H22N2O4/c1-31-29-27(30)26-24-5-3-2-4-22(24)12-15-25(26)33-17-16-32-23-13-10-21(11-14-23)20-8-6-19(18-28)7-9-20/h2-15H,16-17H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50149204

(CHEMBL323618 | N-Phenyl-N'-(1,2,3,4-tetrahydro-acr...)Show InChI InChI=1S/C26H33N3/c1(2-11-19-27-21-13-5-4-6-14-21)3-12-20-28-26-22-15-7-9-17-24(22)29-25-18-10-8-16-23(25)26/h4-7,9,13-15,17,27H,1-3,8,10-12,16,18-20H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase in rat brain |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Acetylcholinesterase in rat brain |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenylosuccinate synthetase isozyme 2
(Homo sapiens (Human)) | BDBM50149203

(CHEMBL323799 | HYDANTOCIDIN-5'-MONOPHOSPHATE | HYD...)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)O[C@@]2(NC(=O)NC2=O)[C@@H]1O |r| Show InChI InChI=1S/C7H11N2O9P/c10-3-2(1-17-19(14,15)16)18-7(4(3)11)5(12)8-6(13)9-7/h2-4,10-11H,1H2,(H2,14,15,16)(H2,8,9,12,13)/t2-,3-,4-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 675 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Adenylosuccinate synthetase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50149227

((3R,4R,5S)-4-Acetylamino-5-amino-3-(3-phenyl-propy...)Show SMILES CC(=O)N[C@@H]1[C@@H](N)CC(=C[C@H]1NCCCc1ccccc1)C(O)=O |c:8| Show InChI InChI=1S/C18H25N3O3/c1-12(22)21-17-15(19)10-14(18(23)24)11-16(17)20-9-5-8-13-6-3-2-4-7-13/h2-4,6-7,11,15-17,20H,5,8-10,19H2,1H3,(H,21,22)(H,23,24)/t15-,16+,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of influenza A viral enzyme neuraminidase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Adenylosuccinate synthetase isozyme 2
(Homo sapiens (Human)) | BDBM50149248

((Formyl-hydroxy-amino)-acetic acid | CHEMBL331373 ...)Show InChI InChI=1S/C3H5NO4/c5-2-4(8)1-3(6)7/h2,8H,1H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Adenylosuccinate synthetase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50149234
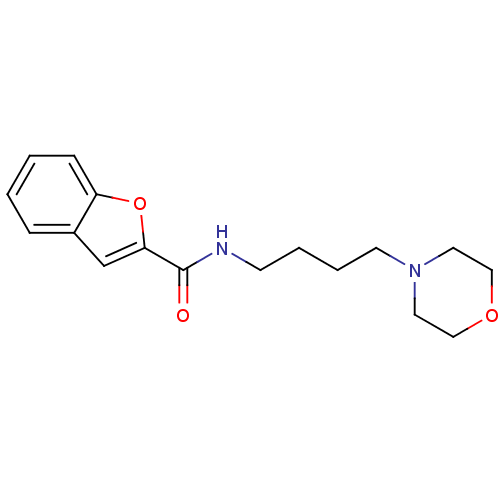
(Benzofuran-2-carboxylic acid (4-morpholin-4-yl-but...)Show InChI InChI=1S/C17H22N2O3/c20-17(16-13-14-5-1-2-6-15(14)22-16)18-7-3-4-8-19-9-11-21-12-10-19/h1-2,5-6,13H,3-4,7-12H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Matrix Metalloprotease-13 (MMP-13) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data