 Found 89 hits Enz. Inhib. hit(s) with all data for entry = 50037406
Found 89 hits Enz. Inhib. hit(s) with all data for entry = 50037406 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171020
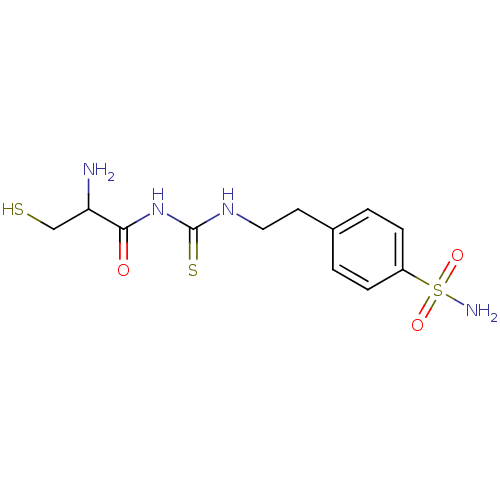
(CHEMBL181082 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C12H18N4O3S3/c13-10(7-20)11(17)16-12(21)15-6-5-8-1-3-9(4-2-8)22(14,18)19/h1-4,10,20H,5-7,13H2,(H2,14,18,19)(H2,15,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171013
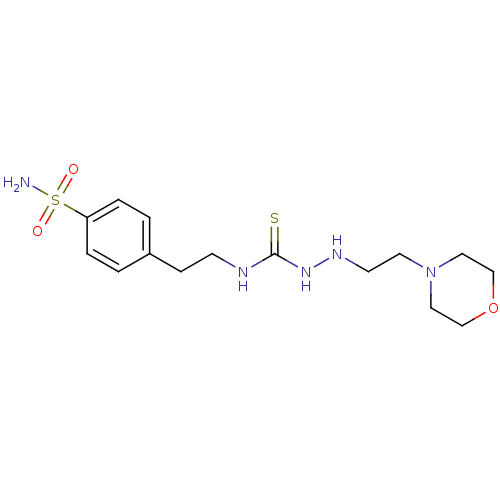
(CHEMBL538564 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C15H25N5O3S2/c16-25(21,22)14-3-1-13(2-4-14)5-6-17-15(24)19-18-7-8-20-9-11-23-12-10-20/h1-4,18H,5-12H2,(H2,16,21,22)(H2,17,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171011
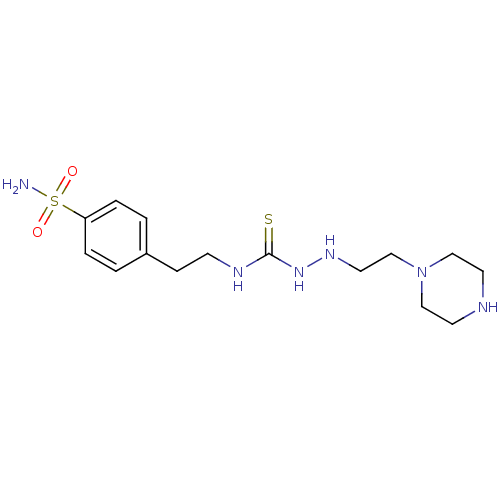
(CHEMBL180177 | Thioureido sulfonamide)Show InChI InChI=1S/C15H26N6O2S2/c16-25(22,23)14-3-1-13(2-4-14)5-6-18-15(24)20-19-9-12-21-10-7-17-8-11-21/h1-4,17,19H,5-12H2,(H2,16,22,23)(H2,18,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171014
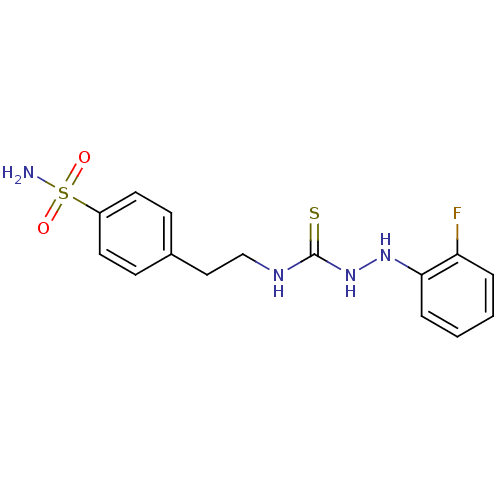
(CHEMBL179355 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C15H17FN4O2S2/c16-13-3-1-2-4-14(13)19-20-15(23)18-10-9-11-5-7-12(8-6-11)24(17,21)22/h1-8,19H,9-10H2,(H2,17,21,22)(H2,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171016
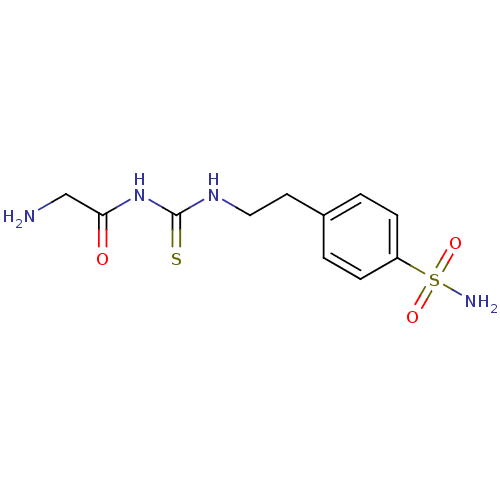
(4-{2-[3-(2-Amino-acetyl)-thioureido]-ethyl}-benzen...)Show InChI InChI=1S/C11H16N4O3S2/c12-7-10(16)15-11(19)14-6-5-8-1-3-9(4-2-8)20(13,17)18/h1-4H,5-7,12H2,(H2,13,17,18)(H2,14,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171023
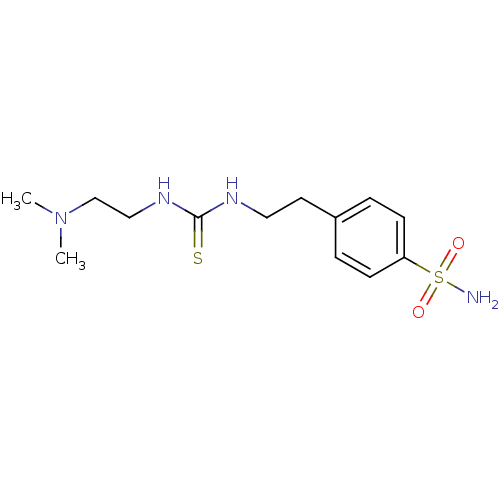
(4-{2-[3-(2-Dimethylamino-ethyl)-thioureido]-ethyl}...)Show InChI InChI=1S/C13H22N4O2S2/c1-17(2)10-9-16-13(20)15-8-7-11-3-5-12(6-4-11)21(14,18)19/h3-6H,7-10H2,1-2H3,(H2,14,18,19)(H2,15,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171025
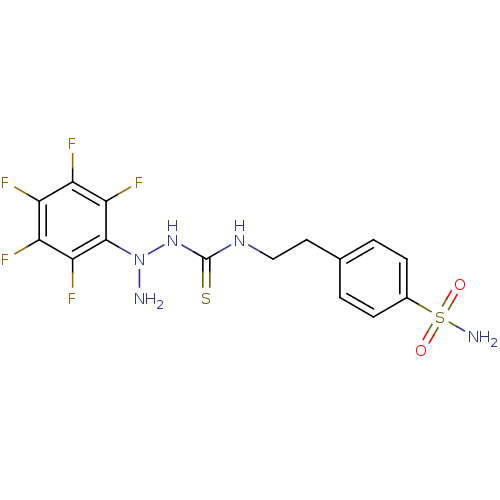
(CHEMBL182197 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NN(NC(=S)NCCc1ccc(cc1)S(N)(=O)=O)c1c(F)c(F)c(F)c(F)c1F Show InChI InChI=1S/C15H14F5N5O2S2/c16-9-10(17)12(19)14(13(20)11(9)18)25(21)24-15(28)23-6-5-7-1-3-8(4-2-7)29(22,26)27/h1-4H,5-6,21H2,(H2,22,26,27)(H2,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171024
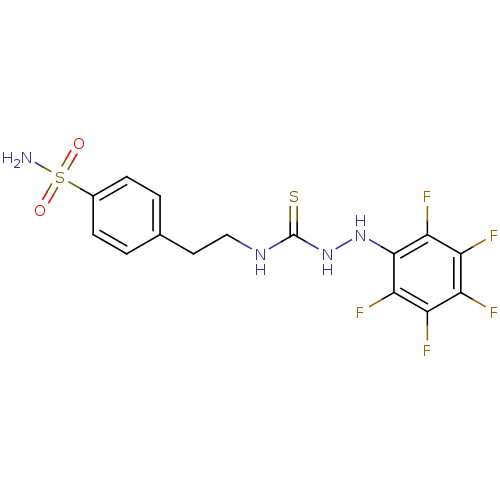
(CHEMBL361955 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)NNc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C15H13F5N4O2S2/c16-9-10(17)12(19)14(13(20)11(9)18)23-24-15(27)22-6-5-7-1-3-8(4-2-7)28(21,25)26/h1-4,23H,5-6H2,(H2,21,25,26)(H2,22,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171012
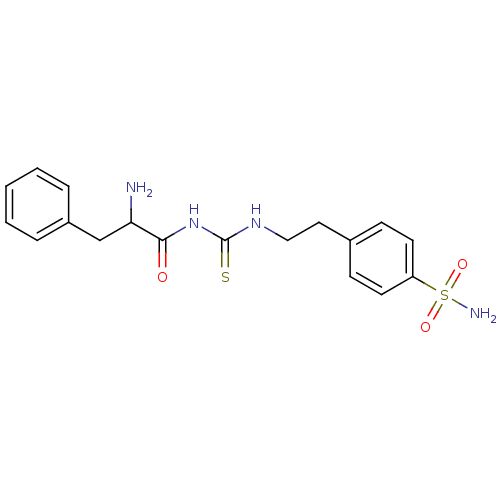
(CHEMBL180506 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NC(Cc1ccccc1)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H22N4O3S2/c19-16(12-14-4-2-1-3-5-14)17(23)22-18(26)21-11-10-13-6-8-15(9-7-13)27(20,24)25/h1-9,16H,10-12,19H2,(H2,20,24,25)(H2,21,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171009
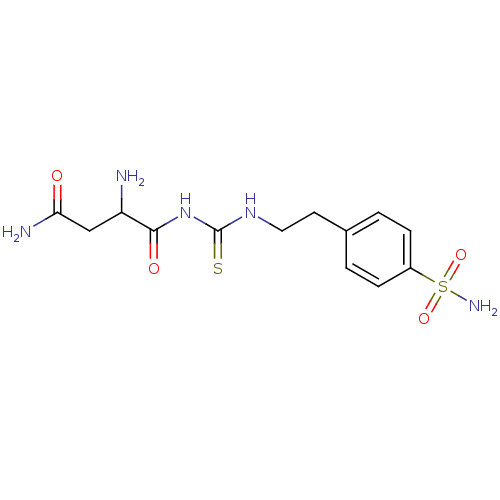
((3R)-3-{2-[({2-[4-(aminosulfonyl)phenyl]ethyl}amin...)Show SMILES NC(CC(N)=O)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C13H19N5O4S2/c14-10(7-11(15)19)12(20)18-13(23)17-6-5-8-1-3-9(4-2-8)24(16,21)22/h1-4,10H,5-7,14H2,(H2,15,19)(H2,16,21,22)(H2,17,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171026
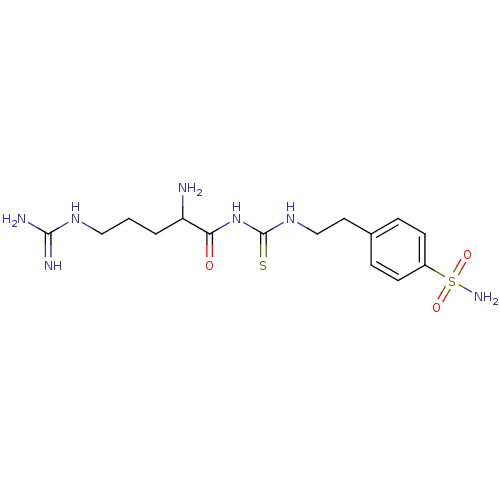
(4-{2-[3-(2-Amino-5-guanidino-pentanoyl)-thioureido...)Show SMILES NC(CCCNC(N)=N)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H25N7O3S2/c16-12(2-1-8-20-14(17)18)13(23)22-15(26)21-9-7-10-3-5-11(6-4-10)27(19,24)25/h3-6,12H,1-2,7-9,16H2,(H4,17,18,20)(H2,19,24,25)(H2,21,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171018
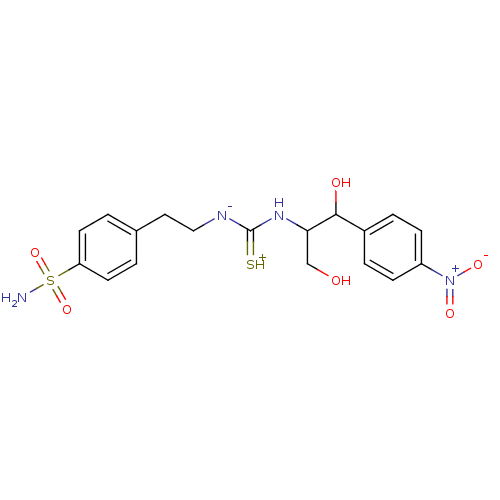
(4-(2-{3-[2-Hydroxy-1-hydroxymethyl-2-(4-nitro-phen...)Show SMILES NS(=O)(=O)c1ccc(CC[N-]C(=[SH+])NC(CO)C(O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22N4O6S2/c19-30(27,28)15-7-1-12(2-8-15)9-10-20-18(29)21-16(11-23)17(24)13-3-5-14(6-4-13)22(25)26/h1-8,16-17,23-24H,9-11H2,(H4,19,20,21,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171020

(CHEMBL181082 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C12H18N4O3S3/c13-10(7-20)11(17)16-12(21)15-6-5-8-1-3-9(4-2-8)22(14,18)19/h1-4,10,20H,5-7,13H2,(H2,14,18,19)(H2,15,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171014

(CHEMBL179355 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C15H17FN4O2S2/c16-13-3-1-2-4-14(13)19-20-15(23)18-10-9-11-5-7-12(8-6-11)24(17,21)22/h1-8,19H,9-10H2,(H2,17,21,22)(H2,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171011

(CHEMBL180177 | Thioureido sulfonamide)Show InChI InChI=1S/C15H26N6O2S2/c16-25(22,23)14-3-1-13(2-4-14)5-6-18-15(24)20-19-9-12-21-10-7-17-8-11-21/h1-4,17,19H,5-12H2,(H2,16,22,23)(H2,18,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171017
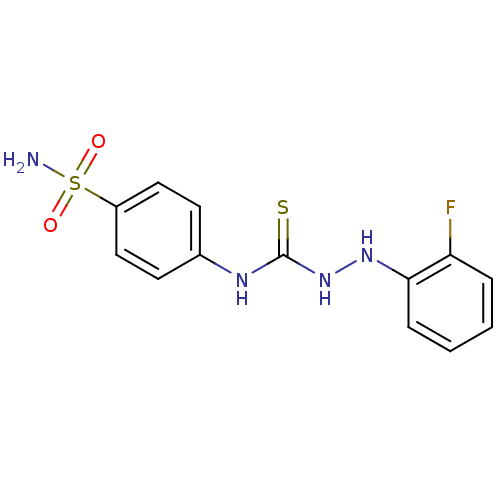
(CHEMBL178403 | N-[4-(aminosulfonyl)phenyl]-2-(2-fl...)Show InChI InChI=1S/C13H13FN4O2S2/c14-11-3-1-2-4-12(11)17-18-13(21)16-9-5-7-10(8-6-9)22(15,19)20/h1-8,17H,(H2,15,19,20)(H2,16,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171016

(4-{2-[3-(2-Amino-acetyl)-thioureido]-ethyl}-benzen...)Show InChI InChI=1S/C11H16N4O3S2/c12-7-10(16)15-11(19)14-6-5-8-1-3-9(4-2-8)20(13,17)18/h1-4H,5-7,12H2,(H2,13,17,18)(H2,14,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171013

(CHEMBL538564 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C15H25N5O3S2/c16-25(21,22)14-3-1-13(2-4-14)5-6-17-15(24)19-18-7-8-20-9-11-23-12-10-20/h1-4,18H,5-12H2,(H2,16,21,22)(H2,17,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171015
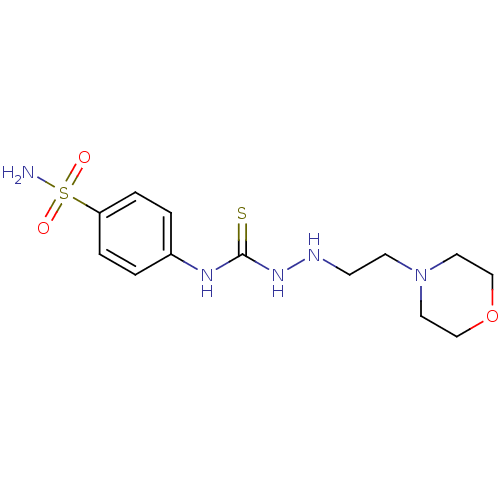
(CHEMBL182135 | N-[4-(aminosulfonyl)phenyl]-2-(2-mo...)Show InChI InChI=1S/C13H21N5O3S2/c14-23(19,20)12-3-1-11(2-4-12)16-13(22)17-15-5-6-18-7-9-21-10-8-18/h1-4,15H,5-10H2,(H2,14,19,20)(H2,16,17,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171025

(CHEMBL182197 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NN(NC(=S)NCCc1ccc(cc1)S(N)(=O)=O)c1c(F)c(F)c(F)c(F)c1F Show InChI InChI=1S/C15H14F5N5O2S2/c16-9-10(17)12(19)14(13(20)11(9)18)25(21)24-15(28)23-6-5-7-1-3-8(4-2-7)29(22,26)27/h1-4H,5-6,21H2,(H2,22,26,27)(H2,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171009

((3R)-3-{2-[({2-[4-(aminosulfonyl)phenyl]ethyl}amin...)Show SMILES NC(CC(N)=O)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C13H19N5O4S2/c14-10(7-11(15)19)12(20)18-13(23)17-6-5-8-1-3-9(4-2-8)24(16,21)22/h1-4,10H,5-7,14H2,(H2,15,19)(H2,16,21,22)(H2,17,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171021
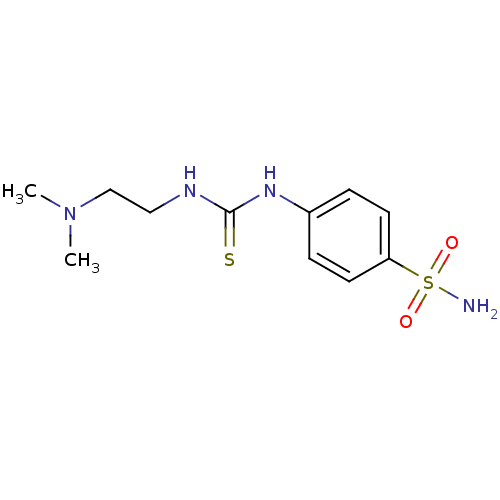
(4-[3-(2-Dimethylamino-ethyl)-thioureido]-benzenesu...)Show InChI InChI=1S/C11H18N4O2S2/c1-15(2)8-7-13-11(18)14-9-3-5-10(6-4-9)19(12,16)17/h3-6H,7-8H2,1-2H3,(H2,12,16,17)(H2,13,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171012

(CHEMBL180506 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NC(Cc1ccccc1)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H22N4O3S2/c19-16(12-14-4-2-1-3-5-14)17(23)22-18(26)21-11-10-13-6-8-15(9-7-13)27(20,24)25/h1-9,16H,10-12,19H2,(H2,20,24,25)(H2,21,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171028
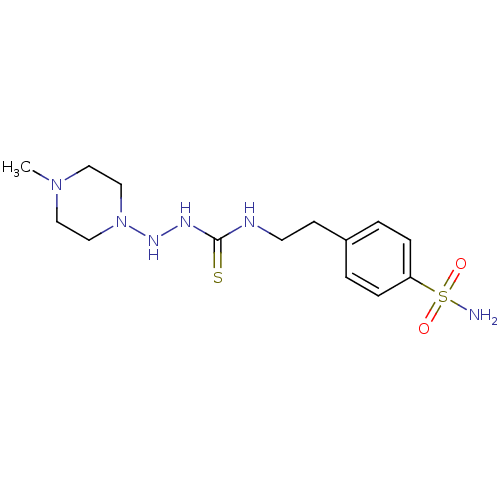
(CHEMBL360254 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C14H24N6O2S2/c1-19-8-10-20(11-9-19)18-17-14(23)16-7-6-12-2-4-13(5-3-12)24(15,21)22/h2-5,18H,6-11H2,1H3,(H2,15,21,22)(H2,16,17,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171010
(CHEMBL362636 | N-[4-(aminosulfonyl)phenyl]-2-(pent...)Show SMILES NN(NC(=S)Nc1ccc(cc1)S(N)(=O)=O)c1c(F)c(F)c(F)c(F)c1F Show InChI InChI=1S/C13H10F5N5O2S2/c14-7-8(15)10(17)12(11(18)9(7)16)23(19)22-13(26)21-5-1-3-6(4-2-5)27(20,24)25/h1-4H,19H2,(H2,20,24,25)(H2,21,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171017

(CHEMBL178403 | N-[4-(aminosulfonyl)phenyl]-2-(2-fl...)Show InChI InChI=1S/C13H13FN4O2S2/c14-11-3-1-2-4-12(11)17-18-13(21)16-9-5-7-10(8-6-9)22(15,19)20/h1-8,17H,(H2,15,19,20)(H2,16,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171015

(CHEMBL182135 | N-[4-(aminosulfonyl)phenyl]-2-(2-mo...)Show InChI InChI=1S/C13H21N5O3S2/c14-23(19,20)12-3-1-11(2-4-12)16-13(22)17-15-5-6-18-7-9-21-10-8-18/h1-4,15H,5-10H2,(H2,14,19,20)(H2,16,17,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171023

(4-{2-[3-(2-Dimethylamino-ethyl)-thioureido]-ethyl}...)Show InChI InChI=1S/C13H22N4O2S2/c1-17(2)10-9-16-13(20)15-8-7-11-3-5-12(6-4-11)21(14,18)19/h3-6H,7-10H2,1-2H3,(H2,14,18,19)(H2,15,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171018

(4-(2-{3-[2-Hydroxy-1-hydroxymethyl-2-(4-nitro-phen...)Show SMILES NS(=O)(=O)c1ccc(CC[N-]C(=[SH+])NC(CO)C(O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22N4O6S2/c19-30(27,28)15-7-1-12(2-8-15)9-10-20-18(29)21-16(11-23)17(24)13-3-5-14(6-4-13)22(25)26/h1-8,16-17,23-24H,9-11H2,(H4,19,20,21,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171026

(4-{2-[3-(2-Amino-5-guanidino-pentanoyl)-thioureido...)Show SMILES NC(CCCNC(N)=N)C(=O)NC(=S)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H25N7O3S2/c16-12(2-1-8-20-14(17)18)13(23)22-15(26)21-9-7-10-3-5-11(6-4-10)27(19,24)25/h3-6,12H,1-2,7-9,16H2,(H4,17,18,20)(H2,19,24,25)(H2,21,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171024

(CHEMBL361955 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)NNc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C15H13F5N4O2S2/c16-9-10(17)12(19)14(13(20)11(9)18)23-24-15(27)22-6-5-7-1-3-8(4-2-7)28(21,25)26/h1-4,23H,5-6H2,(H2,21,25,26)(H2,22,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM11640
(3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)NNc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H9F5N4O2S2/c14-7-8(15)10(17)12(11(18)9(7)16)21-22-13(25)20-5-1-3-6(4-2-5)26(19,23)24/h1-4,21H,(H2,19,23,24)(H2,20,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171010

(CHEMBL362636 | N-[4-(aminosulfonyl)phenyl]-2-(pent...)Show SMILES NN(NC(=S)Nc1ccc(cc1)S(N)(=O)=O)c1c(F)c(F)c(F)c(F)c1F Show InChI InChI=1S/C13H10F5N5O2S2/c14-7-8(15)10(17)12(11(18)9(7)16)23(19)22-13(26)21-5-1-3-6(4-2-5)27(20,24)25/h1-4H,19H2,(H2,20,24,25)(H2,21,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50171017

(CHEMBL178403 | N-[4-(aminosulfonyl)phenyl]-2-(2-fl...)Show InChI InChI=1S/C13H13FN4O2S2/c14-11-3-1-2-4-12(11)17-18-13(21)16-9-5-7-10(8-6-9)22(15,19)20/h1-8,17H,(H2,15,19,20)(H2,16,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50171030
(4-{3-[2-Hydroxy-1-hydroxymethyl-2-(4-nitro-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(NC(=[SH+])[N-]C(CO)C(O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C16H18N4O6S2/c17-28(25,26)13-7-3-11(4-8-13)18-16(27)19-14(9-21)15(22)10-1-5-12(6-2-10)20(23)24/h1-8,14-15,21-22H,9H2,(H4,17,18,19,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50171020

(CHEMBL181082 | N-{2-[4-(aminosulfonyl)phenyl]ethyl...)Show InChI InChI=1S/C12H18N4O3S3/c13-10(7-20)11(17)16-12(21)15-6-5-8-1-3-9(4-2-8)22(14,18)19/h1-4,10,20H,5-7,13H2,(H2,14,18,19)(H2,15,16,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11640

(3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)NNc2c(F)c(F)c(F)c(F)c2F)cc1 Show InChI InChI=1S/C13H9F5N4O2S2/c14-7-8(15)10(17)12(11(18)9(7)16)21-22-13(25)20-5-1-3-6(4-2-5)26(19,23)24/h1-4,21H,(H2,19,23,24)(H2,20,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171030

(4-{3-[2-Hydroxy-1-hydroxymethyl-2-(4-nitro-phenyl)...)Show SMILES NS(=O)(=O)c1ccc(NC(=[SH+])[N-]C(CO)C(O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C16H18N4O6S2/c17-28(25,26)13-7-3-11(4-8-13)18-16(27)19-14(9-21)15(22)10-1-5-12(6-2-10)20(23)24/h1-8,14-15,21-22H,9H2,(H4,17,18,19,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50171021

(4-[3-(2-Dimethylamino-ethyl)-thioureido]-benzenesu...)Show InChI InChI=1S/C11H18N4O2S2/c1-15(2)8-7-13-11(18)14-9-3-5-10(6-4-9)19(12,16)17/h3-6H,7-8H2,1-2H3,(H2,12,16,17)(H2,13,14,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3821-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.054
BindingDB Entry DOI: 10.7270/Q2H132SW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data