 Found 13 hits Enz. Inhib. hit(s) with all data for entry = 50037702
Found 13 hits Enz. Inhib. hit(s) with all data for entry = 50037702 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601
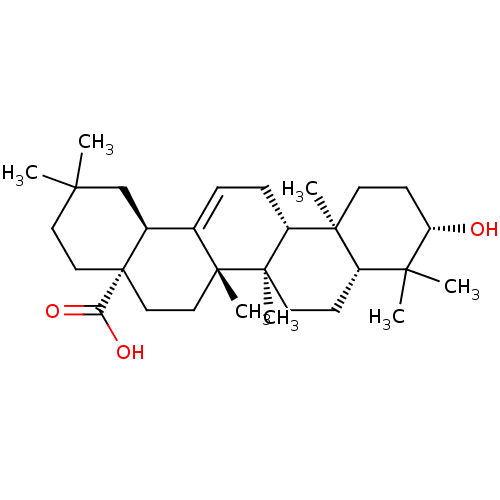
(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694
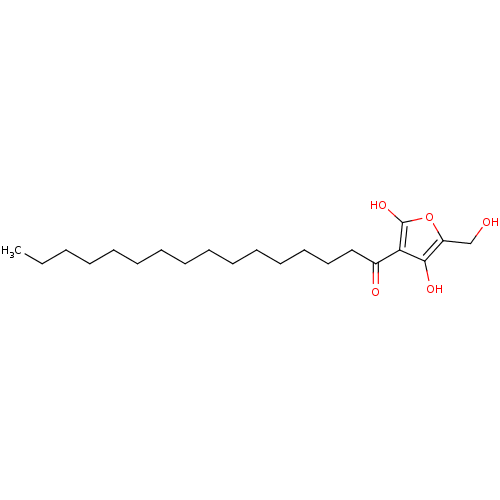
((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185123
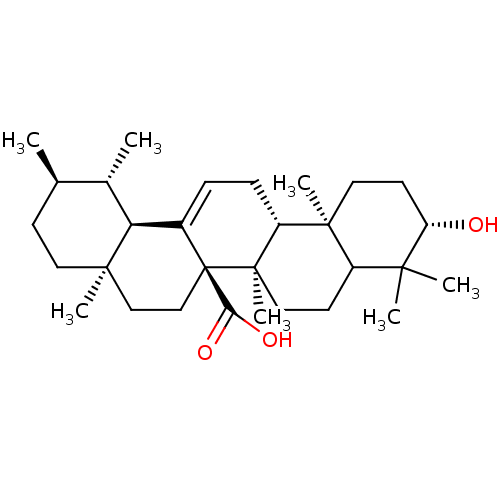
(3beta-hydroxyurs-12-en-27-oic acid | CHEMBL380467)Show SMILES C[C@@H]1CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2[C@H]1C |c:12| Show InChI InChI=1S/C30H48O3/c1-18-10-13-27(5)16-17-30(25(32)33)20(24(27)19(18)2)8-9-22-28(6)14-12-23(31)26(3,4)21(28)11-15-29(22,30)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21?,22-,23+,24+,27-,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185131
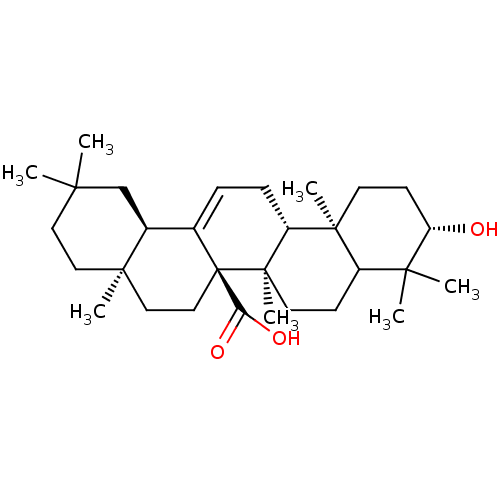
(3beta-hydroxyolean-12-en-27-oic acid | CHEMBL21097...)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1 |c:13| Show InChI InChI=1S/C30H48O3/c1-25(2)14-15-27(5)16-17-30(24(32)33)19(20(27)18-25)8-9-22-28(6)12-11-23(31)26(3,4)21(28)10-13-29(22,30)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22+,23-,27+,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50370716

(CHEMBL1081157)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1 |r,c:13| Show InChI InChI=1S/C30H46O3/c1-25(2)14-15-27(5)16-17-30(24(32)33)19(20(27)18-25)8-9-22-28(6)12-11-23(31)26(3,4)21(28)10-13-29(22,30)7/h8,20-22H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,27+,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185122

(3beta-acetoxyolean-12-en-27-oic acid | CHEMBL20863...)Show SMILES CC(=O)O[C@H]1CC[C@@]2(C)C(CC[C@]3(C)[C@@H]2CC=C2[C@@H]4CC(C)(C)CC[C@]4(C)CC[C@@]32C(O)=O)C1(C)C |t:17| Show InChI InChI=1S/C32H50O4/c1-20(33)36-25-12-13-30(7)23(28(25,4)5)11-14-31(8)24(30)10-9-21-22-19-27(2,3)15-16-29(22,6)17-18-32(21,31)26(34)35/h9,22-25H,10-19H2,1-8H3,(H,34,35)/t22-,23?,24+,25-,29+,30-,31+,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185120
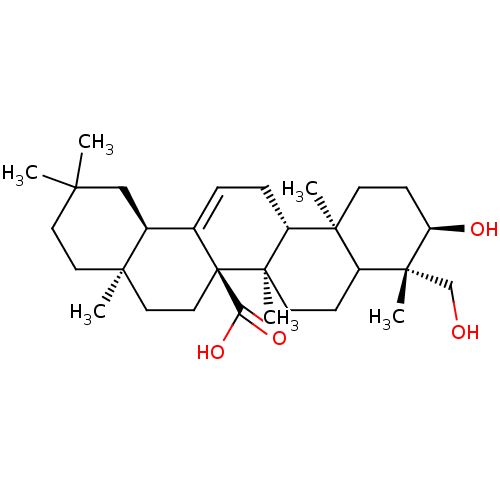
(3alpha,24-dihydroxyolean-12-en-27-oic acid | CHEMB...)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)C5CC[C@@]34C)[C@@H]2C1 |c:13| Show InChI InChI=1S/C30H48O4/c1-25(2)13-14-26(3)15-16-30(24(33)34)19(20(26)17-25)7-8-22-27(4)11-10-23(32)28(5,18-31)21(27)9-12-29(22,30)6/h7,20-23,31-32H,8-18H2,1-6H3,(H,33,34)/t20-,21?,22+,23+,26+,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185125

(3beta,6beta-dihydroxyolean-12-en-27-oic acid | CHE...)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)C5[C@H](O)C[C@@]34C)[C@@H]2C1 |c:13| Show InChI InChI=1S/C30H48O4/c1-25(2)12-13-27(5)14-15-30(24(33)34)18(19(27)16-25)8-9-21-28(6)11-10-22(32)26(3,4)23(28)20(31)17-29(21,30)7/h8,19-23,31-32H,9-17H2,1-7H3,(H,33,34)/t19-,20+,21+,22-,23?,27+,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM23195
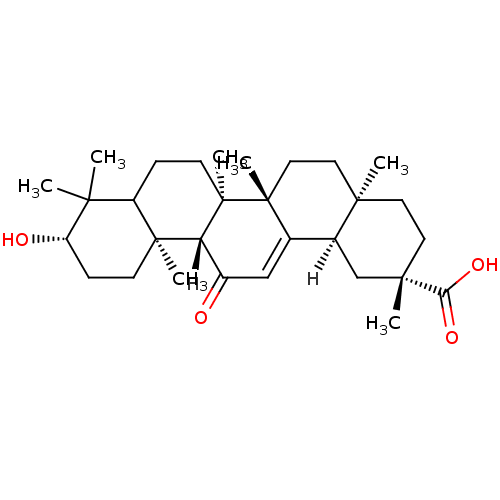
((2S,4aS,6aS,6bR,10S,12aS,12bR,14bR)-10-hydroxy-2,4...)Show SMILES [H][C@@]12C[C@](C)(CC[C@]1(C)CC[C@]1(C)C2=CC(=O)[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)C3CC[C@@]12C)C(O)=O |r,t:15| Show InChI InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21?,22-,23+,26+,27-,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185124

(CHEMBL381707 | methyl 3beta-hydroxyolean-12-en-28-...)Show SMILES COC(=O)[C@@]12CCC3C(=CC[C@H]4[C@@]3(C)CCC3C(C)(C)[C@@H](O)CC[C@]43C)[C@@H]1CC(C)(C)CC2 |c:8| Show InChI InChI=1S/C30H48O3/c1-26(2)16-17-30(25(32)33-7)15-10-20-19(21(30)18-26)8-9-23-28(20,5)13-11-22-27(3,4)24(31)12-14-29(22,23)6/h8,20-24,31H,9-18H2,1-7H3/t20?,21-,22?,23-,24-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185128
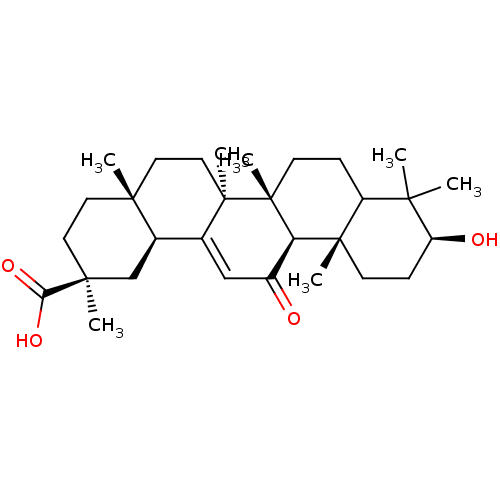
(18alpha-Glycyrrhetic acid | CHEMBL378653)Show SMILES CC1(C)[C@@H](O)CC[C@@]2(C)C1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O |t:19| Show InChI InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21?,22+,23-,26-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185130
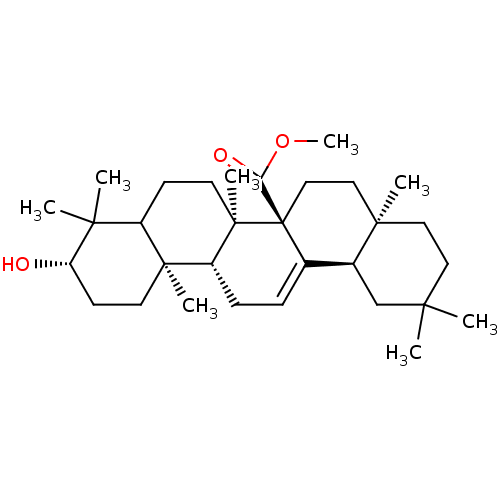
(CHEMBL207494 | methyl3beta-hydroxyolean-12-en-27-o...)Show SMILES COC(=O)[C@@]12CC[C@@]3(C)CCC(C)(C)C[C@H]3C1=CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)C3CC[C@@]21C |c:18| Show InChI InChI=1S/C31H50O3/c1-26(2)15-16-28(5)17-18-31(25(33)34-8)20(21(28)19-26)9-10-23-29(6)13-12-24(32)27(3,4)22(29)11-14-30(23,31)7/h9,21-24,32H,10-19H2,1-8H3/t21-,22?,23+,24-,28+,29-,30+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185127
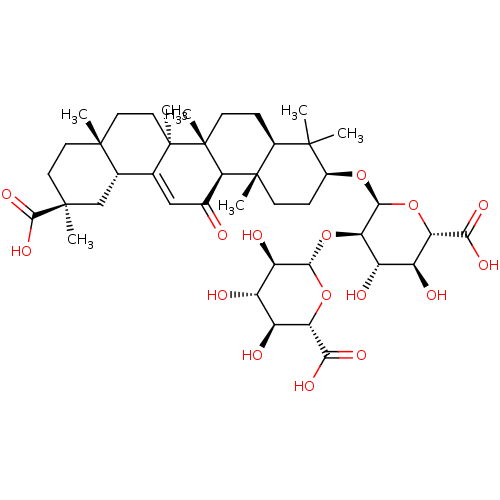
((3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en...)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)O[C@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O)C(O)=O |r,t:18| Show InChI InChI=1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-30(26(47)25(46)29(57-35)33(51)52)58-34-27(48)23(44)24(45)28(56-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25-,26-,27+,28-,29-,30+,31+,34-,35-,38+,39-,40-,41+,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data