

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50097579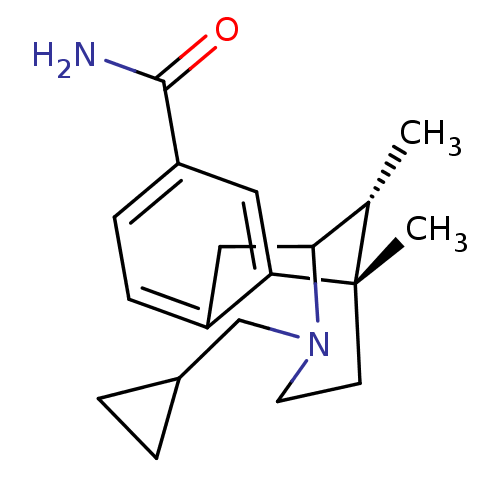 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194266 ((-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194266 ((-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194264 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50097579 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194266 ((-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50097586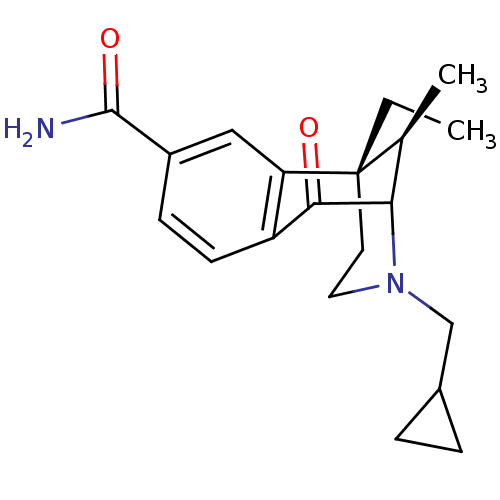 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-11-methyl-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194264 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194272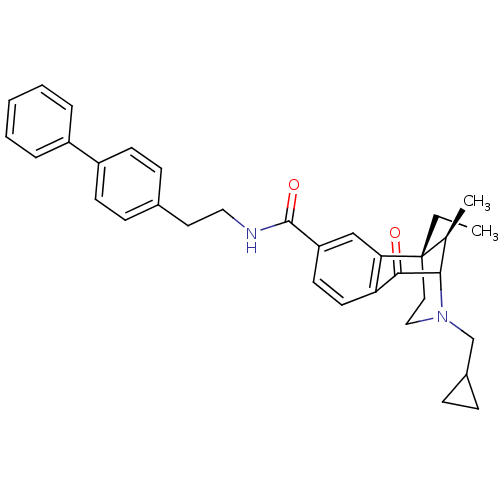 ((+/-)-3-(cyclopropylmethyl)-6-ethyl-1,2,3,4,5,6-he...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194271 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194264 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50097586 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-11-methyl-1-o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194265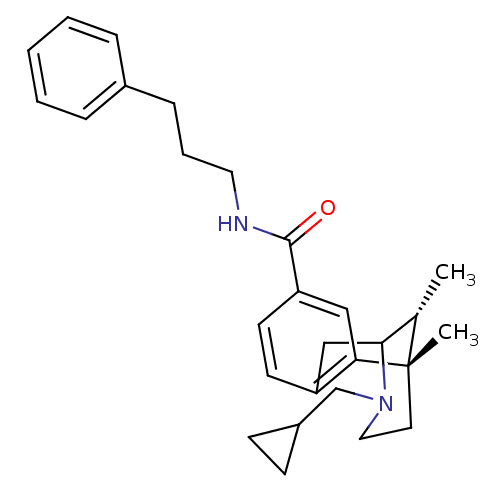 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194270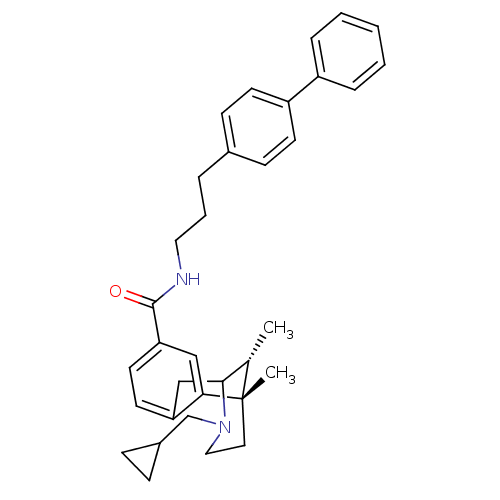 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194265 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194272 ((+/-)-3-(cyclopropylmethyl)-6-ethyl-1,2,3,4,5,6-he...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194271 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194272 ((+/-)-3-(cyclopropylmethyl)-6-ethyl-1,2,3,4,5,6-he...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50097579 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194270 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194267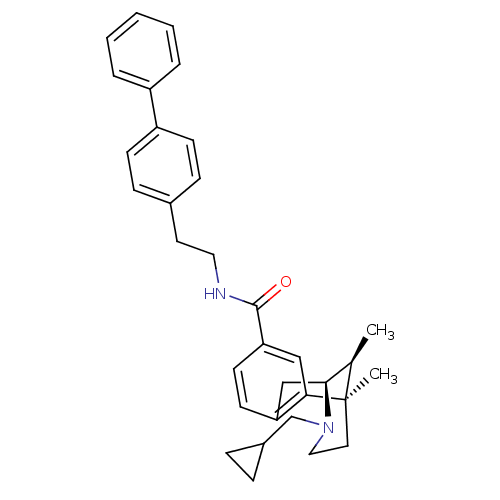 ((+)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194268 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194267 ((+)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194267 ((+)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50370757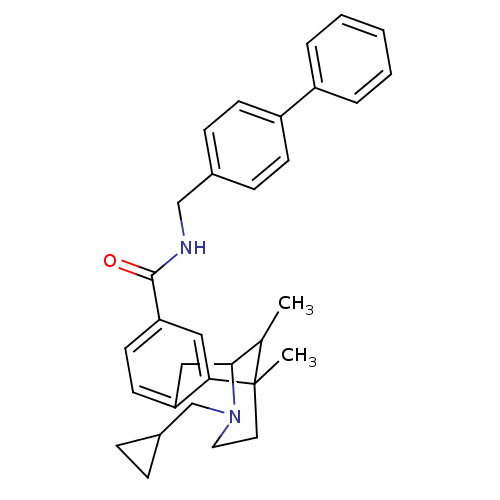 (CHEMBL1162933) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194268 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194268 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194269 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50097586 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-11-methyl-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50370757 (CHEMBL1162933) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194269 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194273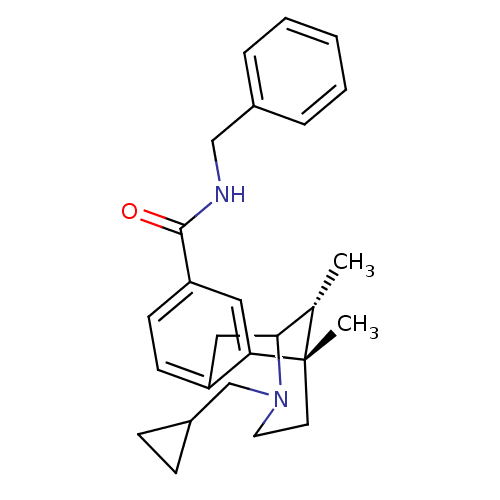 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194273 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194265 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194271 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194270 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194269 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50370757 (CHEMBL1162933) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50194273 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50097577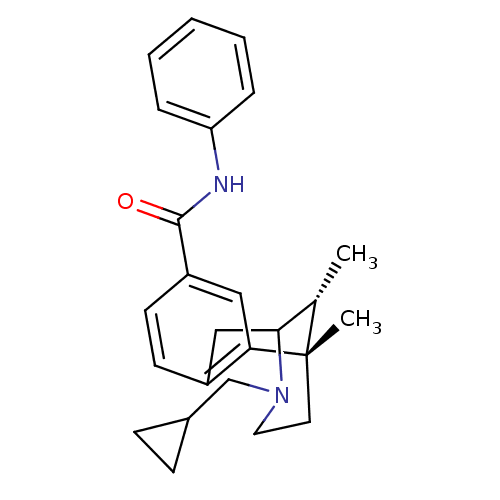 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50097577 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50097577 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50097579 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50097579 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194264 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50097579 ((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human kappa opioid receptor expressed in CHO cells assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50039029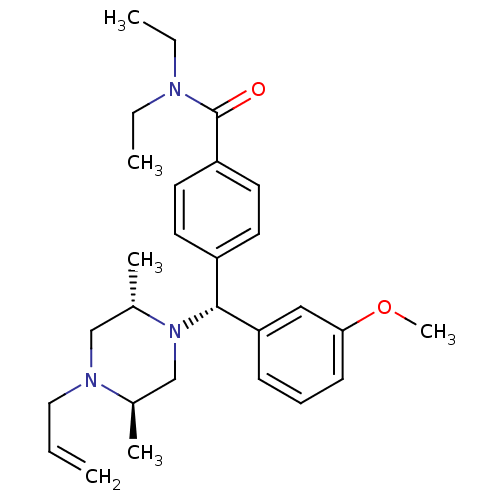 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50194264 ((+/-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human kappa opioid receptor expressed in CHO cells assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296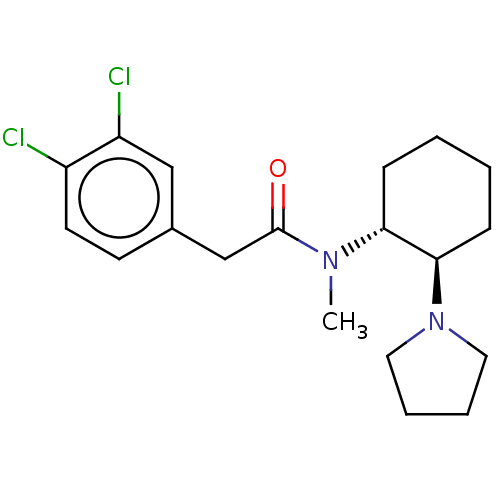 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50194266 ((-)-3-(cyclopropylmethyl)-1,2,3,4,5,6-hexahydro-ci...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Antagonist activity against human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 49: 5635-9 (2006) Article DOI: 10.1021/jm060278n BindingDB Entry DOI: 10.7270/Q208663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |