 Found 88 hits Enz. Inhib. hit(s) with all data for entry = 50037828
Found 88 hits Enz. Inhib. hit(s) with all data for entry = 50037828 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(RAT) | BDBM50205313
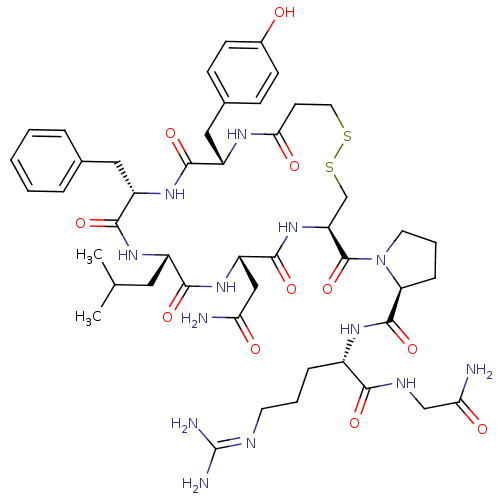
(CHEMBL265859 | d[Leu4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N13O11S2/c1-26(2)20-31-41(66)58-34(23-37(48)62)44(69)59-35(46(71)60-18-7-11-36(60)45(70)55-30(10-6-17-52-47(50)51)40(65)53-24-38(49)63)25-73-72-19-16-39(64)54-32(22-28-12-14-29(61)15-13-28)42(67)57-33(43(68)56-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,61H,6-7,10-11,16-25H2,1-2H3,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,70)(H,56,68)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205305
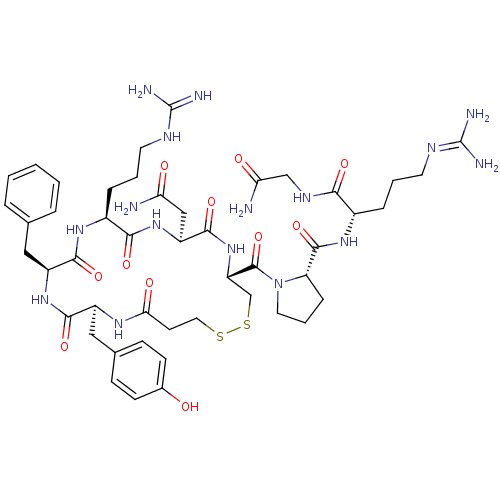
(CHEMBL375324 | d[Arg4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C47H68N16O11S2/c48-36(65)23-33-43(72)62-34(45(74)63-19-6-11-35(63)44(73)59-29(9-4-17-54-46(50)51)39(68)56-24-37(49)66)25-76-75-20-16-38(67)57-31(22-27-12-14-28(64)15-13-27)41(70)60-32(21-26-7-2-1-3-8-26)42(71)58-30(40(69)61-33)10-5-18-55-47(52)53/h1-3,7-8,12-15,29-35,64H,4-6,9-11,16-25H2,(H2,48,65)(H2,49,66)(H,56,68)(H,57,67)(H,58,71)(H,59,73)(H,60,70)(H,61,69)(H,62,72)(H4,50,51,54)(H4,52,53,55)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205309
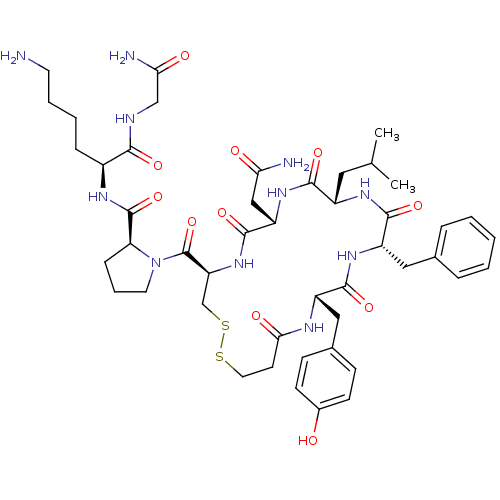
(CHEMBL412972 | d[Leu4,Lys8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C47H67N11O11S2/c1-27(2)21-32-42(64)56-35(24-38(49)60)45(67)57-36(47(69)58-19-8-12-37(58)46(68)53-31(11-6-7-18-48)41(63)51-25-39(50)61)26-71-70-20-17-40(62)52-33(23-29-13-15-30(59)16-14-29)43(65)55-34(44(66)54-32)22-28-9-4-3-5-10-28/h3-5,9-10,13-16,27,31-37,59H,6-8,11-12,17-26,48H2,1-2H3,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,68)(H,54,66)(H,55,65)(H,56,64)(H,57,67)/t31-,32-,33-,34-,35-,36-,37-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205296

(CHEMBL385739 | d[Arg4,Dab8]VP)Show SMILES NCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N14O11S2/c46-16-14-29(38(64)52-23-36(48)62)55-43(69)34-9-5-18-59(34)44(70)33-24-72-71-19-15-37(63)53-30(21-26-10-12-27(60)13-11-26)40(66)56-31(20-25-6-2-1-3-7-25)41(67)54-28(8-4-17-51-45(49)50)39(65)57-32(22-35(47)61)42(68)58-33/h1-3,6-7,10-13,28-34,60H,4-5,8-9,14-24,46H2,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,67)(H,55,69)(H,56,66)(H,57,65)(H,58,68)(H4,49,50,51)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205291
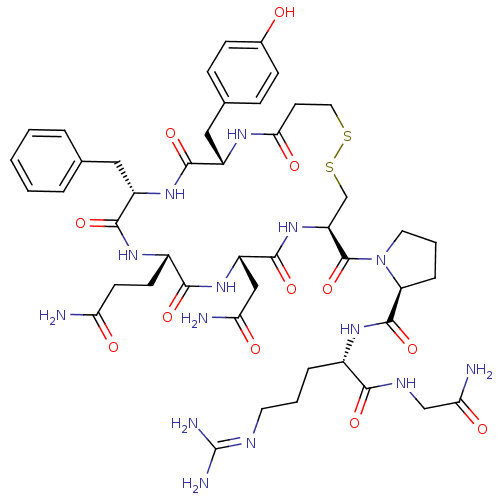
((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205305

(CHEMBL375324 | d[Arg4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C47H68N16O11S2/c48-36(65)23-33-43(72)62-34(45(74)63-19-6-11-35(63)44(73)59-29(9-4-17-54-46(50)51)39(68)56-24-37(49)66)25-76-75-20-16-38(67)57-31(22-27-12-14-28(64)15-13-27)41(70)60-32(21-26-7-2-1-3-8-26)42(71)58-30(40(69)61-33)10-5-18-55-47(52)53/h1-3,7-8,12-15,29-35,64H,4-6,9-11,16-25H2,(H2,48,65)(H2,49,66)(H,56,68)(H,57,67)(H,58,71)(H,59,73)(H,60,70)(H,61,69)(H,62,72)(H4,50,51,54)(H4,52,53,55)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205297
(CHEMBL412973 | d[Leu4,Dab8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCN)C(=O)NCC(N)=O Show InChI InChI=1S/C45H63N11O11S2/c1-25(2)19-30-40(62)54-33(22-36(47)58)43(65)55-34(45(67)56-17-6-9-35(56)44(66)51-29(14-16-46)39(61)49-23-37(48)59)24-69-68-18-15-38(60)50-31(21-27-10-12-28(57)13-11-27)41(63)53-32(42(64)52-30)20-26-7-4-3-5-8-26/h3-5,7-8,10-13,25,29-35,57H,6,9,14-24,46H2,1-2H3,(H2,47,58)(H2,48,59)(H,49,61)(H,50,60)(H,51,66)(H,52,64)(H,53,63)(H,54,62)(H,55,65)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205300
(CHEMBL221436 | d[Val4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205301
(CHEMBL375188 | d[Leu4,Orn8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N11O11S2/c1-26(2)20-31-41(63)55-34(23-37(48)59)44(66)56-35(46(68)57-18-7-11-36(57)45(67)52-30(10-6-17-47)40(62)50-24-38(49)60)25-70-69-19-16-39(61)51-32(22-28-12-14-29(58)15-13-28)42(64)54-33(43(65)53-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,58H,6-7,10-11,16-25,47H2,1-2H3,(H2,48,59)(H2,49,60)(H,50,62)(H,51,61)(H,52,67)(H,53,65)(H,54,64)(H,55,63)(H,56,66)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205304
(CHEMBL435323 | dVDAVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30+,31+,32+,33+,34+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205300

(CHEMBL221436 | d[Val4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205308
((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29+,30+,31+,32+,33+,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205303

(CHEMBL373968 | d[Leu4,Dap8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CN)C(=O)NCC(N)=O Show InChI InChI=1S/C44H61N11O11S2/c1-24(2)17-28-39(61)52-31(20-35(46)57)42(64)54-33(44(66)55-15-6-9-34(55)43(65)53-32(21-45)38(60)48-22-36(47)58)23-68-67-16-14-37(59)49-29(19-26-10-12-27(56)13-11-26)40(62)51-30(41(63)50-28)18-25-7-4-3-5-8-25/h3-5,7-8,10-13,24,28-34,56H,6,9,14-23,45H2,1-2H3,(H2,46,57)(H2,47,58)(H,48,60)(H,49,59)(H,50,63)(H,51,62)(H,52,61)(H,53,65)(H,54,64)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205311
(CHEMBL375096 | d[Orn4]AVP)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H66N14O11S2/c47-17-4-9-30-40(66)58-33(23-36(48)62)43(69)59-34(45(71)60-19-6-11-35(60)44(70)56-29(10-5-18-52-46(50)51)39(65)53-24-37(49)63)25-73-72-20-16-38(64)54-31(22-27-12-14-28(61)15-13-27)41(67)57-32(42(68)55-30)21-26-7-2-1-3-8-26/h1-3,7-8,12-15,29-35,61H,4-6,9-11,16-25,47H2,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205306
(CHEMBL375323 | d[Orn4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C46H66N12O11S2/c47-18-5-4-10-30(40(63)51-25-38(50)61)54-45(68)36-12-7-20-58(36)46(69)35-26-71-70-21-17-39(62)52-32(23-28-13-15-29(59)16-14-28)42(65)55-33(22-27-8-2-1-3-9-27)43(66)53-31(11-6-19-48)41(64)56-34(24-37(49)60)44(67)57-35/h1-3,8-9,13-16,30-36,59H,4-7,10-12,17-26,47-48H2,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,66)(H,54,68)(H,55,65)(H,56,64)(H,57,67)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205298
(CHEMBL375325 | d[Arg4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C47H68N14O11S2/c48-18-5-4-10-30(40(66)54-25-38(50)64)57-45(71)36-12-7-20-61(36)46(72)35-26-74-73-21-17-39(65)55-32(23-28-13-15-29(62)16-14-28)42(68)58-33(22-27-8-2-1-3-9-27)43(69)56-31(11-6-19-53-47(51)52)41(67)59-34(24-37(49)63)44(70)60-35/h1-3,8-9,13-16,30-36,62H,4-7,10-12,17-26,48H2,(H2,49,63)(H2,50,64)(H,54,66)(H,55,65)(H,56,69)(H,57,71)(H,58,68)(H,59,67)(H,60,70)(H4,51,52,53)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205292

(CHEMBL385068 | d[Arg4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C46H66N14O11S2/c47-17-4-9-29(39(65)53-24-37(49)63)56-44(70)35-11-6-19-60(35)45(71)34-25-73-72-20-16-38(64)54-31(22-27-12-14-28(61)15-13-27)41(67)57-32(21-26-7-2-1-3-8-26)42(68)55-30(10-5-18-52-46(50)51)40(66)58-33(23-36(48)62)43(69)59-34/h1-3,7-8,12-15,29-35,61H,4-6,9-11,16-25,47H2,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205291

((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205307

(CHEMBL412742 | d[Cha4,Dab8]VP)Show SMILES NCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C48H67N11O11S2/c49-19-17-32(42(64)52-26-40(51)62)54-47(69)38-12-7-20-59(38)48(70)37-27-72-71-21-18-41(63)53-33(24-30-13-15-31(60)16-14-30)43(65)55-34(22-28-8-3-1-4-9-28)44(66)56-35(23-29-10-5-2-6-11-29)45(67)57-36(25-39(50)61)46(68)58-37/h1,3-4,8-9,13-16,29,32-38,60H,2,5-7,10-12,17-27,49H2,(H2,50,61)(H2,51,62)(H,52,64)(H,53,63)(H,54,69)(H,55,65)(H,56,66)(H,57,67)(H,58,68)/t32-,33-,34-,35-,36-,37-,38-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205310

(CHEMBL219272 | d[Orn4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N12O11S2/c46-17-4-9-29(39(62)50-24-37(49)60)53-44(67)35-11-6-19-57(35)45(68)34-25-70-69-20-16-38(61)51-31(22-27-12-14-28(58)15-13-27)41(64)54-32(21-26-7-2-1-3-8-26)42(65)52-30(10-5-18-47)40(63)55-33(23-36(48)59)43(66)56-34/h1-3,7-8,12-15,29-35,58H,4-6,9-11,16-25,46-47H2,(H2,48,59)(H2,49,60)(H,50,62)(H,51,61)(H,52,65)(H,53,67)(H,54,64)(H,55,63)(H,56,66)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205291

((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM35667
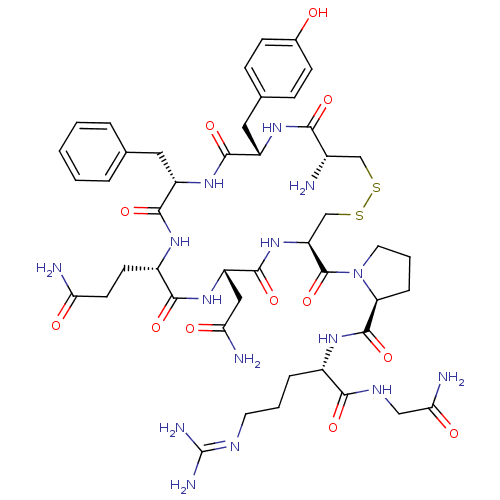
(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205312

(CHEMBL265858 | d[Cha4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C50H71N13O11S2/c51-40(65)26-37-47(72)62-38(49(74)63-21-8-14-39(63)48(73)58-33(13-7-20-55-50(53)54)43(68)56-27-41(52)66)28-76-75-22-19-42(67)57-34(25-31-15-17-32(64)18-16-31)44(69)59-35(23-29-9-3-1-4-10-29)45(70)60-36(46(71)61-37)24-30-11-5-2-6-12-30/h1,3-4,9-10,15-18,30,33-39,64H,2,5-8,11-14,19-28H2,(H2,51,65)(H2,52,66)(H,56,68)(H,57,67)(H,58,73)(H,59,69)(H,60,70)(H,61,71)(H,62,72)(H4,53,54,55)/t33-,34-,35-,36-,37-,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205296

(CHEMBL385739 | d[Arg4,Dab8]VP)Show SMILES NCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N14O11S2/c46-16-14-29(38(64)52-23-36(48)62)55-43(69)34-9-5-18-59(34)44(70)33-24-72-71-19-15-37(63)53-30(21-26-10-12-27(60)13-11-26)40(66)56-31(20-25-6-2-1-3-7-25)41(67)54-28(8-4-17-51-45(49)50)39(65)57-32(22-35(47)61)42(68)58-33/h1-3,6-7,10-13,28-34,60H,4-5,8-9,14-24,46H2,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,67)(H,55,69)(H,56,66)(H,57,65)(H,58,68)(H4,49,50,51)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205293

(CHEMBL221485 | d[Cha4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C50H71N11O11S2/c51-21-8-7-14-34(44(66)54-28-42(53)64)56-49(71)40-15-9-22-61(40)50(72)39-29-74-73-23-20-43(65)55-35(26-32-16-18-33(62)19-17-32)45(67)57-36(24-30-10-3-1-4-11-30)46(68)58-37(25-31-12-5-2-6-13-31)47(69)59-38(27-41(52)63)48(70)60-39/h1,3-4,10-11,16-19,31,34-40,62H,2,5-9,12-15,20-29,51H2,(H2,52,63)(H2,53,64)(H,54,66)(H,55,65)(H,56,71)(H,57,67)(H,58,68)(H,59,69)(H,60,70)/t34-,35-,36-,37-,38-,39-,40-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205295
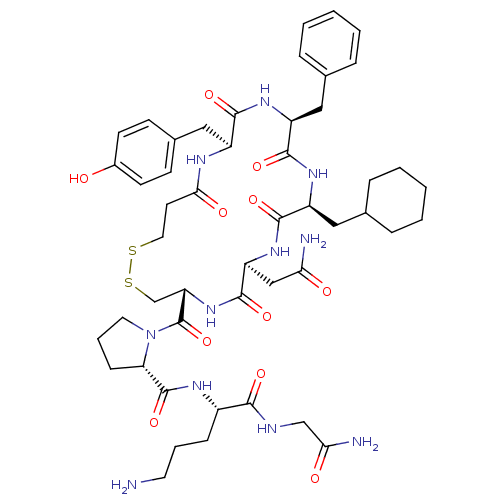
(CHEMBL263090 | d[Cha4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C49H69N11O11S2/c50-20-7-13-33(43(65)53-27-41(52)63)55-48(70)39-14-8-21-60(39)49(71)38-28-73-72-22-19-42(64)54-34(25-31-15-17-32(61)18-16-31)44(66)56-35(23-29-9-3-1-4-10-29)45(67)57-36(24-30-11-5-2-6-12-30)46(68)58-37(26-40(51)62)47(69)59-38/h1,3-4,9-10,15-18,30,33-39,61H,2,5-8,11-14,19-28,50H2,(H2,51,62)(H2,52,63)(H,53,65)(H,54,64)(H,55,70)(H,56,66)(H,57,67)(H,58,68)(H,59,69)/t33-,34-,35-,36-,37-,38-,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205292

(CHEMBL385068 | d[Arg4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C46H66N14O11S2/c47-17-4-9-29(39(65)53-24-37(49)63)56-44(70)35-11-6-19-60(35)45(71)34-25-73-72-20-16-38(64)54-31(22-27-12-14-28(61)15-13-27)41(67)57-32(21-26-7-2-1-3-8-26)42(68)55-30(10-5-18-52-46(50)51)40(66)58-33(23-36(48)62)43(69)59-34/h1-3,7-8,12-15,29-35,61H,4-6,9-11,16-25,47H2,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205313

(CHEMBL265859 | d[Leu4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N13O11S2/c1-26(2)20-31-41(66)58-34(23-37(48)62)44(69)59-35(46(71)60-18-7-11-36(60)45(70)55-30(10-6-17-52-47(50)51)40(65)53-24-38(49)63)25-73-72-19-16-39(64)54-32(22-28-12-14-29(61)15-13-28)42(67)57-33(43(68)56-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,61H,6-7,10-11,16-25H2,1-2H3,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,70)(H,56,68)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205311

(CHEMBL375096 | d[Orn4]AVP)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H66N14O11S2/c47-17-4-9-30-40(66)58-33(23-36(48)62)43(69)59-34(45(71)60-19-6-11-35(60)44(70)56-29(10-5-18-52-46(50)51)39(65)53-24-37(49)63)25-73-72-20-16-38(64)54-31(22-27-12-14-28(61)15-13-27)41(67)57-32(42(68)55-30)21-26-7-2-1-3-8-26/h1-3,7-8,12-15,29-35,61H,4-6,9-11,16-25,47H2,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205298

(CHEMBL375325 | d[Arg4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C47H68N14O11S2/c48-18-5-4-10-30(40(66)54-25-38(50)64)57-45(71)36-12-7-20-61(36)46(72)35-26-74-73-21-17-39(65)55-32(23-28-13-15-29(62)16-14-28)42(68)58-33(22-27-8-2-1-3-9-27)43(69)56-31(11-6-19-53-47(51)52)41(67)59-34(24-37(49)63)44(70)60-35/h1-3,8-9,13-16,30-36,62H,4-7,10-12,17-26,48H2,(H2,49,63)(H2,50,64)(H,54,66)(H,55,65)(H,56,69)(H,57,71)(H,58,68)(H,59,67)(H,60,70)(H4,51,52,53)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205299
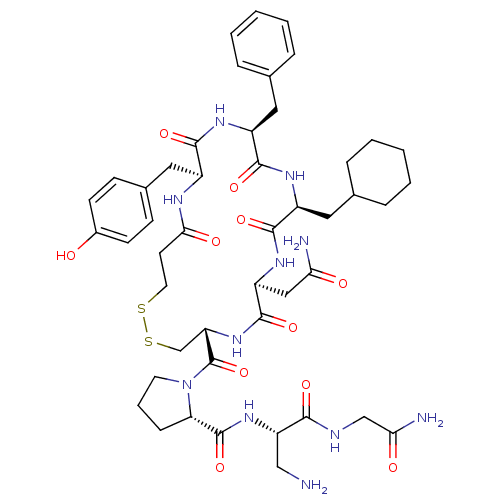
(CHEMBL375187 | d[Cha4,Dap8]VP)Show SMILES NC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CC2CCCCC2)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C47H65N11O11S2/c48-24-35(41(63)51-25-39(50)61)56-46(68)37-12-7-18-58(37)47(69)36-26-71-70-19-17-40(62)52-31(22-29-13-15-30(59)16-14-29)42(64)53-32(20-27-8-3-1-4-9-27)43(65)54-33(21-28-10-5-2-6-11-28)44(66)55-34(23-38(49)60)45(67)57-36/h1,3-4,8-9,13-16,28,31-37,59H,2,5-7,10-12,17-26,48H2,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,64)(H,54,65)(H,55,66)(H,56,68)(H,57,67)/t31-,32-,33-,34-,35-,36-,37-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205291

((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205312

(CHEMBL265858 | d[Cha4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C50H71N13O11S2/c51-40(65)26-37-47(72)62-38(49(74)63-21-8-14-39(63)48(73)58-33(13-7-20-55-50(53)54)43(68)56-27-41(52)66)28-76-75-22-19-42(67)57-34(25-31-15-17-32(64)18-16-31)44(69)59-35(23-29-9-3-1-4-10-29)45(70)60-36(46(71)61-37)24-30-11-5-2-6-12-30/h1,3-4,9-10,15-18,30,33-39,64H,2,5-8,11-14,19-28H2,(H2,51,65)(H2,52,66)(H,56,68)(H,57,67)(H,58,73)(H,59,69)(H,60,70)(H,61,71)(H,62,72)(H4,53,54,55)/t33-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205305

(CHEMBL375324 | d[Arg4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C47H68N16O11S2/c48-36(65)23-33-43(72)62-34(45(74)63-19-6-11-35(63)44(73)59-29(9-4-17-54-46(50)51)39(68)56-24-37(49)66)25-76-75-20-16-38(67)57-31(22-27-12-14-28(64)15-13-27)41(70)60-32(21-26-7-2-1-3-8-26)42(71)58-30(40(69)61-33)10-5-18-55-47(52)53/h1-3,7-8,12-15,29-35,64H,4-6,9-11,16-25H2,(H2,48,65)(H2,49,66)(H,56,68)(H,57,67)(H,58,71)(H,59,73)(H,60,70)(H,61,69)(H,62,72)(H4,50,51,54)(H4,52,53,55)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205306

(CHEMBL375323 | d[Orn4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C46H66N12O11S2/c47-18-5-4-10-30(40(63)51-25-38(50)61)54-45(68)36-12-7-20-58(36)46(69)35-26-71-70-21-17-39(62)52-32(23-28-13-15-29(59)16-14-28)42(65)55-33(22-27-8-2-1-3-9-27)43(66)53-31(11-6-19-48)41(64)56-34(24-37(49)60)44(67)57-35/h1-3,8-9,13-16,30-36,59H,4-7,10-12,17-26,47-48H2,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,66)(H,54,68)(H,55,65)(H,56,64)(H,57,67)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205296

(CHEMBL385739 | d[Arg4,Dab8]VP)Show SMILES NCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N14O11S2/c46-16-14-29(38(64)52-23-36(48)62)55-43(69)34-9-5-18-59(34)44(70)33-24-72-71-19-15-37(63)53-30(21-26-10-12-27(60)13-11-26)40(66)56-31(20-25-6-2-1-3-7-25)41(67)54-28(8-4-17-51-45(49)50)39(65)57-32(22-35(47)61)42(68)58-33/h1-3,6-7,10-13,28-34,60H,4-5,8-9,14-24,46H2,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,67)(H,55,69)(H,56,66)(H,57,65)(H,58,68)(H4,49,50,51)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205310

(CHEMBL219272 | d[Orn4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N12O11S2/c46-17-4-9-29(39(62)50-24-37(49)60)53-44(67)35-11-6-19-57(35)45(68)34-25-70-69-20-16-38(61)51-31(22-27-12-14-28(58)15-13-27)41(64)54-32(21-26-7-2-1-3-8-26)42(65)52-30(10-5-18-47)40(63)55-33(23-36(48)59)43(66)56-34/h1-3,7-8,12-15,29-35,58H,4-6,9-11,16-25,46-47H2,(H2,48,59)(H2,49,60)(H,50,62)(H,51,61)(H,52,65)(H,53,67)(H,54,64)(H,55,63)(H,56,66)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205297

(CHEMBL412973 | d[Leu4,Dab8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCN)C(=O)NCC(N)=O Show InChI InChI=1S/C45H63N11O11S2/c1-25(2)19-30-40(62)54-33(22-36(47)58)43(65)55-34(45(67)56-17-6-9-35(56)44(66)51-29(14-16-46)39(61)49-23-37(48)59)24-69-68-18-15-38(60)50-31(21-27-10-12-28(57)13-11-27)41(63)53-32(42(64)52-30)20-26-7-4-3-5-8-26/h3-5,7-8,10-13,25,29-35,57H,6,9,14-24,46H2,1-2H3,(H2,47,58)(H2,48,59)(H,49,61)(H,50,60)(H,51,66)(H,52,64)(H,53,63)(H,54,62)(H,55,65)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205306

(CHEMBL375323 | d[Orn4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C46H66N12O11S2/c47-18-5-4-10-30(40(63)51-25-38(50)61)54-45(68)36-12-7-20-58(36)46(69)35-26-71-70-21-17-39(62)52-32(23-28-13-15-29(59)16-14-28)42(65)55-33(22-27-8-2-1-3-9-27)43(66)53-31(11-6-19-48)41(64)56-34(24-37(49)60)44(67)57-35/h1-3,8-9,13-16,30-36,59H,4-7,10-12,17-26,47-48H2,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,66)(H,54,68)(H,55,65)(H,56,64)(H,57,67)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205301

(CHEMBL375188 | d[Leu4,Orn8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N11O11S2/c1-26(2)20-31-41(63)55-34(23-37(48)59)44(66)56-35(46(68)57-18-7-11-36(57)45(67)52-30(10-6-17-47)40(62)50-24-38(49)60)25-70-69-19-16-39(61)51-32(22-28-12-14-29(58)15-13-28)42(64)54-33(43(65)53-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,58H,6-7,10-11,16-25,47H2,1-2H3,(H2,48,59)(H2,49,60)(H,50,62)(H,51,61)(H,52,67)(H,53,65)(H,54,64)(H,55,63)(H,56,66)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205300

(CHEMBL221436 | d[Val4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205309

(CHEMBL412972 | d[Leu4,Lys8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C47H67N11O11S2/c1-27(2)21-32-42(64)56-35(24-38(49)60)45(67)57-36(47(69)58-19-8-12-37(58)46(68)53-31(11-6-7-18-48)41(63)51-25-39(50)61)26-71-70-20-17-40(62)52-33(23-29-13-15-30(59)16-14-29)43(65)55-34(44(66)54-32)22-28-9-4-3-5-10-28/h3-5,9-10,13-16,27,31-37,59H,6-8,11-12,17-26,48H2,1-2H3,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,68)(H,54,66)(H,55,65)(H,56,64)(H,57,67)/t31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205292

(CHEMBL385068 | d[Arg4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C46H66N14O11S2/c47-17-4-9-29(39(65)53-24-37(49)63)56-44(70)35-11-6-19-60(35)45(71)34-25-73-72-20-16-38(64)54-31(22-27-12-14-28(61)15-13-27)41(67)57-32(21-26-7-2-1-3-8-26)42(68)55-30(10-5-18-52-46(50)51)40(66)58-33(23-36(48)62)43(69)59-34/h1-3,7-8,12-15,29-35,61H,4-6,9-11,16-25,47H2,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205294

(CHEMBL414074 | d[D-3-Pal2]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2cccnc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C45H63N15O11S2/c46-34(61)13-12-28-39(66)58-31(21-35(47)62)42(69)59-32(44(71)60-17-6-11-33(60)43(70)56-27(10-5-16-52-45(49)50)38(65)53-23-36(48)63)24-73-72-18-14-37(64)54-29(20-26-9-4-15-51-22-26)40(67)57-30(41(68)55-28)19-25-7-2-1-3-8-25/h1-4,7-9,15,22,27-33H,5-6,10-14,16-21,23-24H2,(H2,46,61)(H2,47,62)(H2,48,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,49,50,52)/t27-,28-,29+,30-,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205310

(CHEMBL219272 | d[Orn4,Orn8]VP)Show SMILES NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N12O11S2/c46-17-4-9-29(39(62)50-24-37(49)60)53-44(67)35-11-6-19-57(35)45(68)34-25-70-69-20-16-38(61)51-31(22-27-12-14-28(58)15-13-27)41(64)54-32(21-26-7-2-1-3-8-26)42(65)52-30(10-5-18-47)40(63)55-33(23-36(48)59)43(66)56-34/h1-3,7-8,12-15,29-35,58H,4-6,9-11,16-25,46-47H2,(H2,48,59)(H2,49,60)(H,50,62)(H,51,61)(H,52,65)(H,53,67)(H,54,64)(H,55,63)(H,56,66)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205308

((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29+,30+,31+,32+,33+,34+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205309

(CHEMBL412972 | d[Leu4,Lys8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C47H67N11O11S2/c1-27(2)21-32-42(64)56-35(24-38(49)60)45(67)57-36(47(69)58-19-8-12-37(58)46(68)53-31(11-6-7-18-48)41(63)51-25-39(50)61)26-71-70-20-17-40(62)52-33(23-29-13-15-30(59)16-14-29)43(65)55-34(44(66)54-32)22-28-9-4-3-5-10-28/h3-5,9-10,13-16,27,31-37,59H,6-8,11-12,17-26,48H2,1-2H3,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,68)(H,54,66)(H,55,65)(H,56,64)(H,57,67)/t31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205294

(CHEMBL414074 | d[D-3-Pal2]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2cccnc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C45H63N15O11S2/c46-34(61)13-12-28-39(66)58-31(21-35(47)62)42(69)59-32(44(71)60-17-6-11-33(60)43(70)56-27(10-5-16-52-45(49)50)38(65)53-23-36(48)63)24-73-72-18-14-37(64)54-29(20-26-9-4-15-51-22-26)40(67)57-30(41(68)55-28)19-25-7-2-1-3-8-25/h1-4,7-9,15,22,27-33H,5-6,10-14,16-21,23-24H2,(H2,46,61)(H2,47,62)(H2,48,63)(H,53,65)(H,54,64)(H,55,68)(H,56,70)(H,57,67)(H,58,66)(H,59,69)(H4,49,50,52)/t27-,28-,29+,30-,31-,32-,33-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205298

(CHEMBL375325 | d[Arg4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C47H68N14O11S2/c48-18-5-4-10-30(40(66)54-25-38(50)64)57-45(71)36-12-7-20-61(36)46(72)35-26-74-73-21-17-39(65)55-32(23-28-13-15-29(62)16-14-28)42(68)58-33(22-27-8-2-1-3-9-27)43(69)56-31(11-6-19-53-47(51)52)41(67)59-34(24-37(49)63)44(70)60-35/h1-3,8-9,13-16,30-36,62H,4-7,10-12,17-26,48H2,(H2,49,63)(H2,50,64)(H,54,66)(H,55,65)(H,56,69)(H,57,71)(H,58,68)(H,59,67)(H,60,70)(H4,51,52,53)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat OT receptor expressed in CHO cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50205298

(CHEMBL375325 | d[Arg4,Lys8]VP)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C47H68N14O11S2/c48-18-5-4-10-30(40(66)54-25-38(50)64)57-45(71)36-12-7-20-61(36)46(72)35-26-74-73-21-17-39(65)55-32(23-28-13-15-29(62)16-14-28)42(68)58-33(22-27-8-2-1-3-9-27)43(69)56-31(11-6-19-53-47(51)52)41(67)59-34(24-37(49)63)44(70)60-35/h1-3,8-9,13-16,30-36,62H,4-7,10-12,17-26,48H2,(H2,49,63)(H2,50,64)(H,54,66)(H,55,65)(H,56,69)(H,57,71)(H,58,68)(H,59,67)(H,60,70)(H4,51,52,53)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V1a receptor in rat liver membrane |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data