 Found 29 hits Enz. Inhib. hit(s) with all data for entry = 50037916
Found 29 hits Enz. Inhib. hit(s) with all data for entry = 50037916 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214370
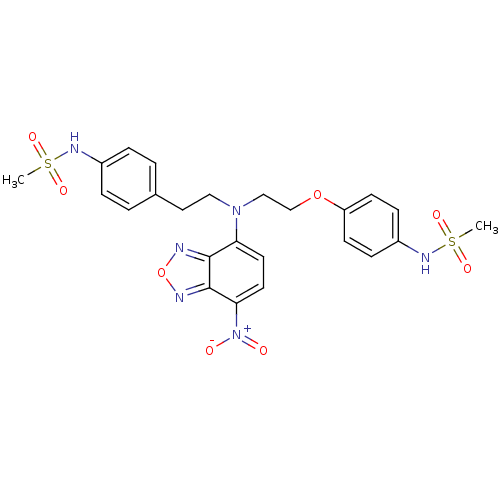
(CHEMBL227134 | N-(4-{2-[[2-(4-methanesulfonylamino...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCOc2ccc(NS(C)(=O)=O)cc2)c2ccc([N+]([O-])=O)c3nonc23)cc1 Show InChI InChI=1S/C24H26N6O8S2/c1-39(33,34)27-18-5-3-17(4-6-18)13-14-29(21-11-12-22(30(31)32)24-23(21)25-38-26-24)15-16-37-20-9-7-19(8-10-20)28-40(2,35)36/h3-12,27-28H,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214364

(11-ethyl-1-(3-{[2-(4-methanesulfonylamino-phenoxy)...)Show SMILES CCN1CCCc2cc3NC4C=C5CCC[N+](CCCC(=O)N(CCOc6ccc(NS(C)(=O)=O)cc6)CCc6ccc(NS(C)(=O)=O)cc6)=C5C=C4Oc3cc12 |w:10.10,c:51,54,t:11| Show InChI InChI=1S/C42H53N6O7S2/c1-4-46-20-5-8-31-26-36-40(28-38(31)46)55-41-29-39-32(27-37(41)43-36)9-6-21-47(39)22-7-10-42(49)48(23-19-30-11-13-33(14-12-30)44-56(2,50)51)24-25-54-35-17-15-34(16-18-35)45-57(3,52)53/h11-18,26-29,37,43-45H,4-10,19-25H2,1-3H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031720
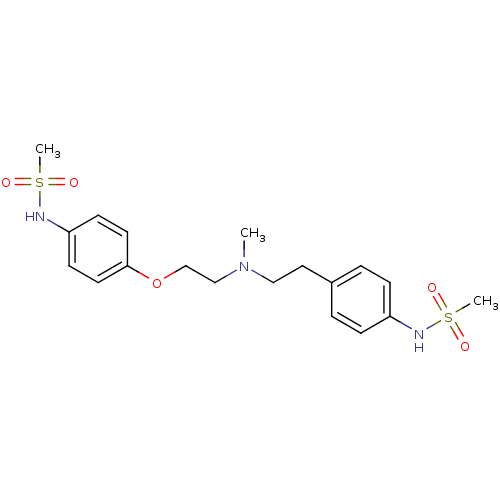
((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...)Show SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214372
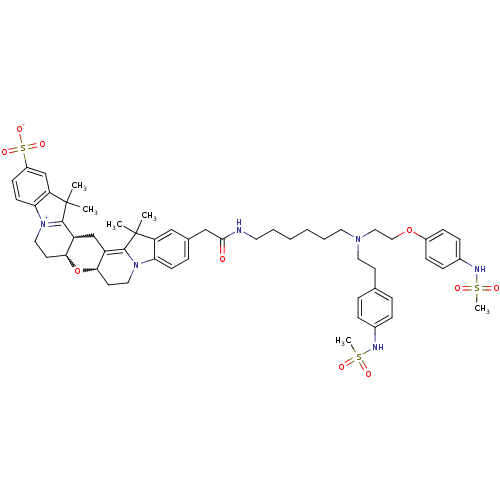
(24-{[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3CC4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,6.5,7.27,c:3,10| Show InChI InChI=1S/C55H70N6O10S3/c1-54(2)45-33-38(13-21-47(45)60-29-24-49-43(52(54)60)36-44-50(71-49)25-30-61-48-22-20-42(74(67,68)69)35-46(48)55(3,4)53(44)61)34-51(62)56-26-9-7-8-10-27-59(28-23-37-11-14-39(15-12-37)57-72(5,63)64)31-32-70-41-18-16-40(17-19-41)58-73(6,65)66/h11-22,33,35,44,49-50,57-58H,7-10,23-32,34,36H2,1-6H3,(H-,56,62,67,68,69) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031720

((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...)Show SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214373
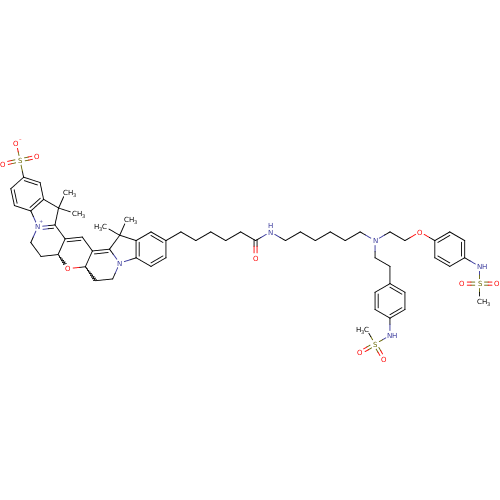
(24-{5-[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CCCCCC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:7.27,26.30,c:3,5,10| Show InChI InChI=1S/C59H76N6O10S3/c1-58(2)49-38-42(18-26-51(49)64-34-29-53-47(56(58)64)40-48-54(75-53)30-35-65-52-27-25-46(78(71,72)73)39-50(52)59(3,4)57(48)65)14-10-9-11-15-55(66)60-31-12-7-8-13-32-63(33-28-41-16-19-43(20-17-41)61-76(5,67)68)36-37-74-45-23-21-44(22-24-45)62-77(6,69)70/h16-27,38-40,53-54,61-62H,7-15,28-37H2,1-6H3,(H-,60,66,71,72,73) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214369

(24-{[(5-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,7.27,c:3,5,10| Show InChI InChI=1S/C54H66N6O10S3/c1-53(2)44-32-37(12-20-46(44)59-28-23-48-42(51(53)59)35-43-49(70-48)24-29-60-47-21-19-41(73(66,67)68)34-45(47)54(3,4)52(43)60)33-50(61)55-25-8-7-9-26-58(27-22-36-10-13-38(14-11-36)56-71(5,62)63)30-31-69-40-17-15-39(16-18-40)57-72(6,64)65/h10-21,32,34-35,48-49,56-57H,7-9,22-31,33H2,1-6H3,(H-,55,61,66,67,68) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214376
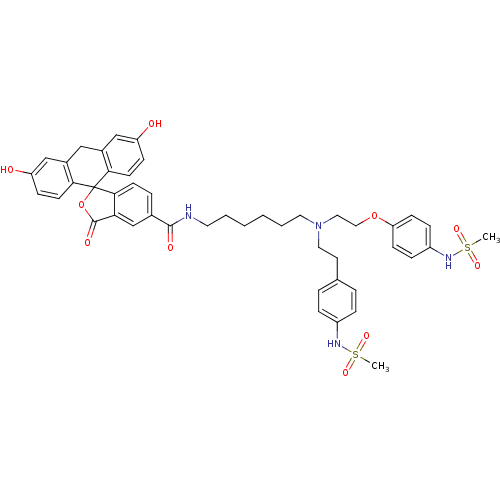
(3,6-dihydroxy-N-(6-{[2-(4-methanesulfonamidophenox...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCCCCCNC(=O)c2ccc3c(c2)C(=O)OC32c3ccc(O)cc3Cc3cc(O)ccc23)CCOc2ccc(NS(C)(=O)=O)cc2)cc1 Show InChI InChI=1S/C46H50N4O10S2/c1-61(55,56)48-35-10-7-31(8-11-35)21-24-50(25-26-59-39-16-12-36(13-17-39)49-62(2,57)58)23-6-4-3-5-22-47-44(53)32-9-18-43-40(30-32)45(54)60-46(43)41-19-14-37(51)28-33(41)27-34-29-38(52)15-20-42(34)46/h7-20,28-30,48-49,51-52H,3-6,21-27H2,1-2H3,(H,47,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214371
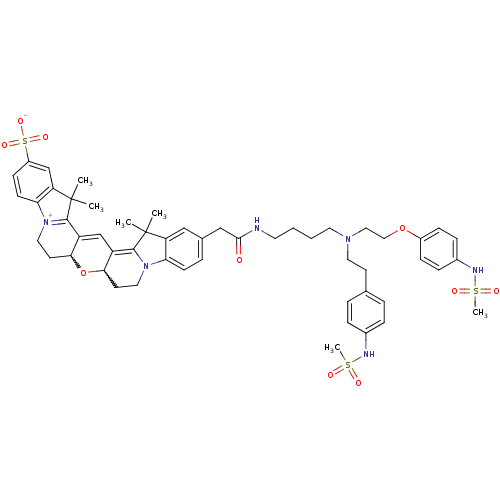
(24-{[(4-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.30,7.27,c:3,5,10| Show InChI InChI=1S/C53H64N6O10S3/c1-52(2)43-31-36(11-19-45(43)58-27-22-47-41(50(52)58)34-42-48(69-47)23-28-59-46-20-18-40(72(65,66)67)33-44(46)53(3,4)51(42)59)32-49(60)54-24-7-8-25-57(26-21-35-9-12-37(13-10-35)55-70(5,61)62)29-30-68-39-16-14-38(15-17-39)56-71(6,63)64/h9-20,31,33-34,47-48,55-56H,7-8,21-30,32H2,1-6H3,(H-,54,60,65,66,67) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930
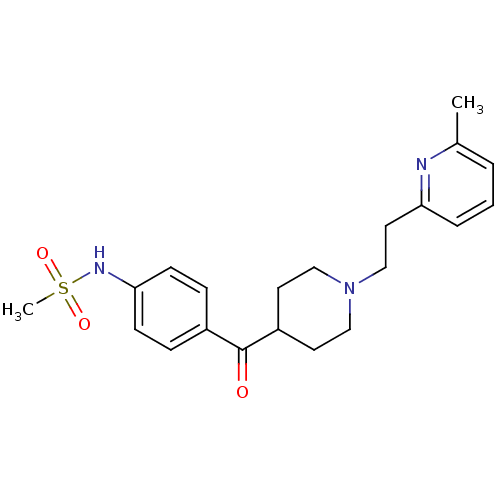
((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214374
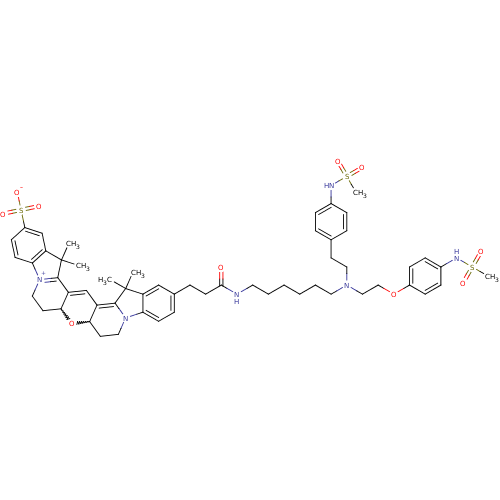
(24-{2-[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CCC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,7.27,c:3,5,10| Show InChI InChI=1S/C56H70N6O10S3/c1-55(2)46-35-39(13-22-48(46)61-31-26-50-44(53(55)61)37-45-51(72-50)27-32-62-49-23-21-43(75(68,69)70)36-47(49)56(3,4)54(45)62)14-24-52(63)57-28-9-7-8-10-29-60(30-25-38-11-15-40(16-12-38)58-73(5,64)65)33-34-71-42-19-17-41(18-20-42)59-74(6,66)67/h11-13,15-23,35-37,50-51,58-59H,7-10,14,24-34H2,1-6H3,(H-,57,63,68,69,70) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930

((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214366
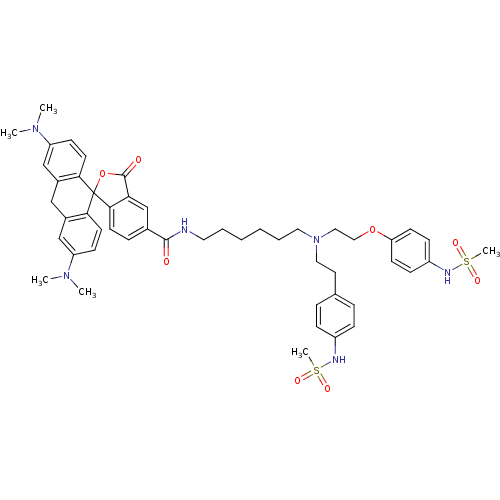
(3,6-bis(dimethylamino)-N-(6-{[2-(4-methanesulfonam...)Show SMILES CN(C)c1ccc2c(Cc3cc(ccc3C22OC(=O)c3cc(ccc23)C(=O)NCCCCCCN(CCOc2ccc(NS(C)(=O)=O)cc2)CCc2ccc(NS(C)(=O)=O)cc2)N(C)C)c1 Show InChI InChI=1S/C50H60N6O8S2/c1-54(2)41-18-23-45-37(32-41)31-38-33-42(55(3)4)19-24-46(38)50(45)47-22-13-36(34-44(47)49(58)64-50)48(57)51-26-9-7-8-10-27-56(28-25-35-11-14-39(15-12-35)52-65(5,59)60)29-30-63-43-20-16-40(17-21-43)53-66(6,61)62/h11-24,32-34,52-53H,7-10,25-31H2,1-6H3,(H,51,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214368
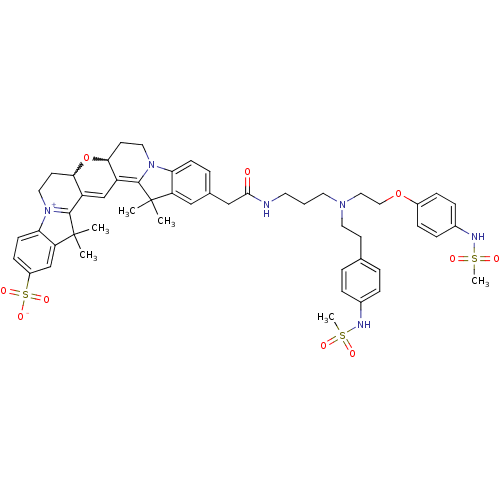
(24-{[(3-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,7.27,c:3,5,10| Show InChI InChI=1S/C52H62N6O10S3/c1-51(2)42-30-35(10-18-44(42)57-26-21-46-40(49(51)57)33-41-47(68-46)22-27-58-45-19-17-39(71(64,65)66)32-43(45)52(3,4)50(41)58)31-48(59)53-23-7-24-56(25-20-34-8-11-36(12-9-34)54-69(5,60)61)28-29-67-38-15-13-37(14-16-38)55-70(6,62)63/h8-19,30,32-33,46-47,54-55H,7,20-29,31H2,1-6H3,(H-,53,59,64,65,66) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM78577
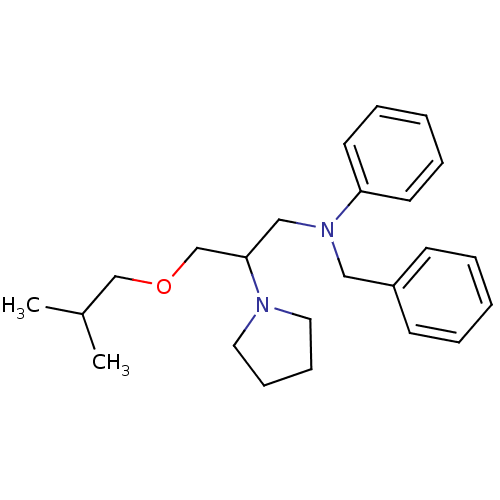
(BEPRIDIL HYDROCHLORIDE | Bepridil | MLS000028456 |...)Show InChI InChI=1S/C24H34N2O/c1-21(2)19-27-20-24(25-15-9-10-16-25)18-26(23-13-7-4-8-14-23)17-22-11-5-3-6-12-22/h3-8,11-14,21,24H,9-10,15-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117922
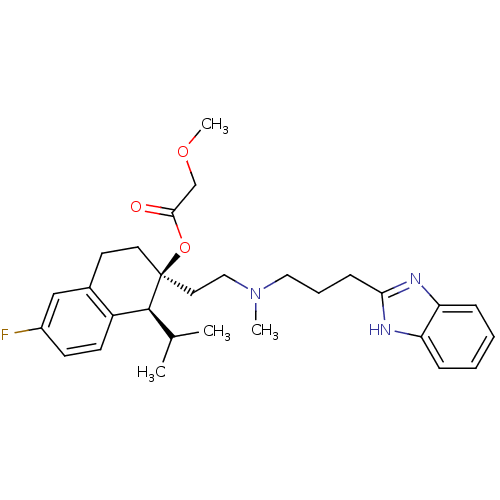
((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...)Show SMILES COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C |r| Show InChI InChI=1S/C29H38FN3O3/c1-20(2)28-23-12-11-22(30)18-21(23)13-14-29(28,36-27(34)19-35-4)15-17-33(3)16-7-10-26-31-24-8-5-6-9-25(24)32-26/h5-6,8-9,11-12,18,20,28H,7,10,13-17,19H2,1-4H3,(H,31,32)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM78577

(BEPRIDIL HYDROCHLORIDE | Bepridil | MLS000028456 |...)Show InChI InChI=1S/C24H34N2O/c1-21(2)19-27-20-24(25-15-9-10-16-25)18-26(23-13-7-4-8-14-23)17-22-11-5-3-6-12-22/h3-8,11-14,21,24H,9-10,15-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117922

((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...)Show SMILES COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C |r| Show InChI InChI=1S/C29H38FN3O3/c1-20(2)28-23-12-11-22(30)18-21(23)13-14-29(28,36-27(34)19-35-4)15-17-33(3)16-7-10-26-31-24-8-5-6-9-25(24)32-26/h5-6,8-9,11-12,18,20,28H,7,10,13-17,19H2,1-4H3,(H,31,32)/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214375
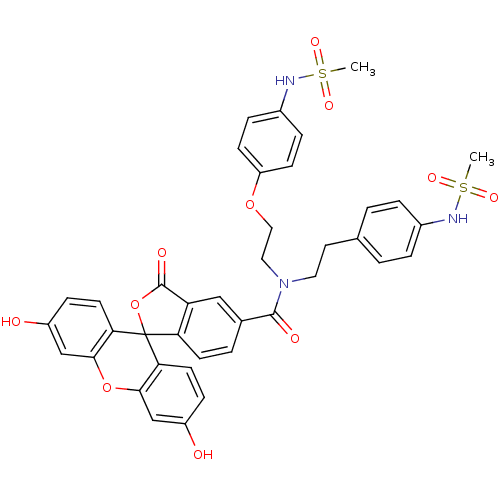
(3',6'-dihydroxy-N-[2-(4-methanesulfonamidophenoxy)...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCOc2ccc(NS(C)(=O)=O)cc2)C(=O)c2ccc3c(c2)C(=O)OC32c3ccc(O)cc3Oc3cc(O)ccc23)cc1 Show InChI InChI=1S/C39H35N3O11S2/c1-54(47,48)40-26-6-3-24(4-7-26)17-18-42(19-20-51-30-12-8-27(9-13-30)41-55(2,49)50)37(45)25-5-14-32-31(21-25)38(46)53-39(32)33-15-10-28(43)22-35(33)52-36-23-29(44)11-16-34(36)39/h3-16,21-23,40-41,43-44H,17-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214363
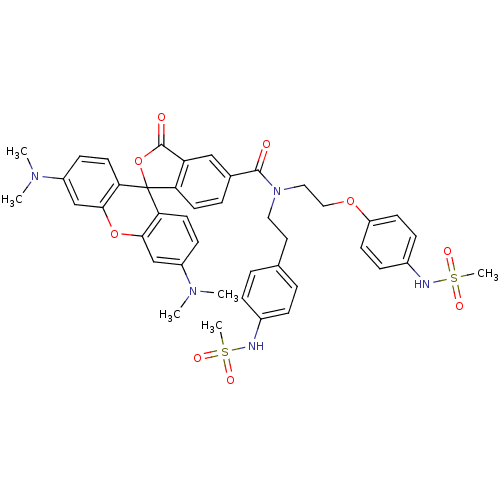
(3',6'-bis(dimethylamino)-N-[2-(4-methanesulfonamid...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3cc(ccc23)C(=O)N(CCOc2ccc(NS(C)(=O)=O)cc2)CCc2ccc(NS(C)(=O)=O)cc2)N(C)C)c1 Show InChI InChI=1S/C43H45N5O9S2/c1-46(2)32-14-19-37-39(26-32)56-40-27-33(47(3)4)15-20-38(40)43(37)36-18-9-29(25-35(36)42(50)57-43)41(49)48(22-21-28-7-10-30(11-8-28)44-58(5,51)52)23-24-55-34-16-12-31(13-17-34)45-59(6,53)54/h7-20,25-27,44-45H,21-24H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214367
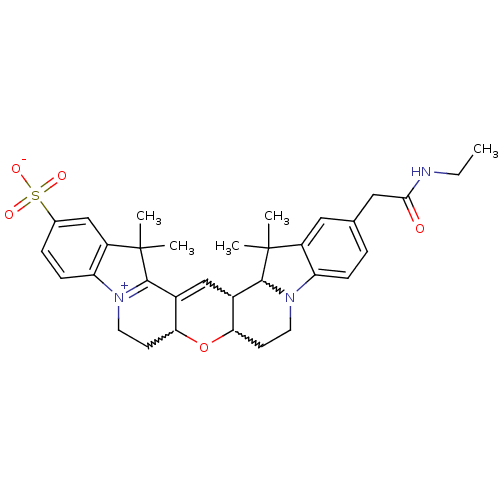
((2-(N-ethylamidomethyl)-6,7,9,10,16,18-hexahydro-1...)Show SMILES CCNC(=O)Cc1ccc2N3CCC4OC5CC[N+]6=C(C5=CC4C3C(C)(C)c2c1)C(C)(C)c1cc(ccc61)S([O-])(=O)=O |w:13.12,23.25,15.15,22.22,c:18,21| Show InChI InChI=1S/C33H39N3O5S/c1-6-34-29(37)16-19-7-9-25-23(15-19)32(2,3)30-21-18-22-28(41-27(21)11-13-35(25)30)12-14-36-26-10-8-20(42(38,39)40)17-24(26)33(4,5)31(22)36/h7-10,15,17-18,21,27-28,30H,6,11-14,16H2,1-5H3,(H-,34,37,38,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214365
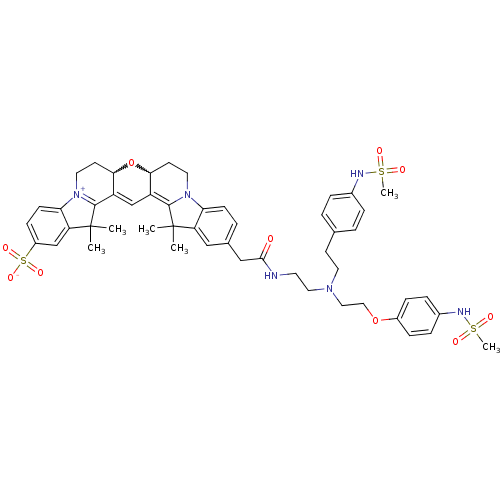
(24-{[(2-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,7.27,c:3,5,10| Show InChI InChI=1S/C51H60N6O10S3/c1-50(2)41-29-34(30-47(58)52-22-26-55(23-19-33-7-10-35(11-8-33)53-68(5,59)60)27-28-66-37-14-12-36(13-15-37)54-69(6,61)62)9-17-43(41)56-24-20-45-39(48(50)56)32-40-46(67-45)21-25-57-44-18-16-38(70(63,64)65)31-42(44)51(3,4)49(40)57/h7-18,29,31-32,45-46,53-54H,19-28,30H2,1-6H3,(H-,52,58,63,64,65) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214370

(CHEMBL227134 | N-(4-{2-[[2-(4-methanesulfonylamino...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCOc2ccc(NS(C)(=O)=O)cc2)c2ccc([N+]([O-])=O)c3nonc23)cc1 Show InChI InChI=1S/C24H26N6O8S2/c1-39(33,34)27-18-5-3-17(4-6-18)13-14-29(21-11-12-22(30(31)32)24-23(21)25-38-26-24)15-16-37-20-9-7-19(8-10-20)28-40(2,35)36/h3-12,27-28H,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Blockade of hERG expressed in HEK293 cells by whole-cell patch clamp method |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031720

((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...)Show SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Blockade of hERG expressed in HEK293 cells by whole-cell patch clamp method |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214372

(24-{[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3CC4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,6.5,7.27,c:3,10| Show InChI InChI=1S/C55H70N6O10S3/c1-54(2)45-33-38(13-21-47(45)60-29-24-49-43(52(54)60)36-44-50(71-49)25-30-61-48-22-20-42(74(67,68)69)35-46(48)55(3,4)53(44)61)34-51(62)56-26-9-7-8-10-27-59(28-23-37-11-14-39(15-12-37)57-72(5,63)64)31-32-70-41-18-16-40(17-19-41)58-73(6,65)66/h11-22,33,35,44,49-50,57-58H,7-10,23-32,34,36H2,1-6H3,(H-,56,62,67,68,69) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Blockade of hERG expressed in HEK293 cells by whole-cell patch clamp method |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data