 Found 103 hits Enz. Inhib. hit(s) with all data for entry = 50037972
Found 103 hits Enz. Inhib. hit(s) with all data for entry = 50037972 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216554
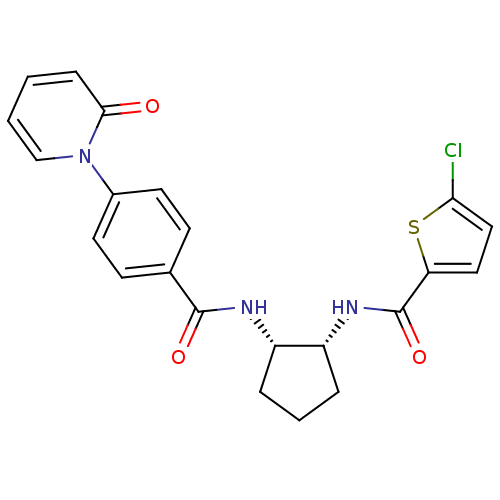
(5-CHLORO-N-((1R,2S)-2-(4-(2-OXOPYRIDIN-1(2H)-YL)BE...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H20ClN3O3S/c23-19-12-11-18(30-19)22(29)25-17-5-3-4-16(17)24-21(28)14-7-9-15(10-8-14)26-13-2-1-6-20(26)27/h1-2,6-13,16-17H,3-5H2,(H,24,28)(H,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216584
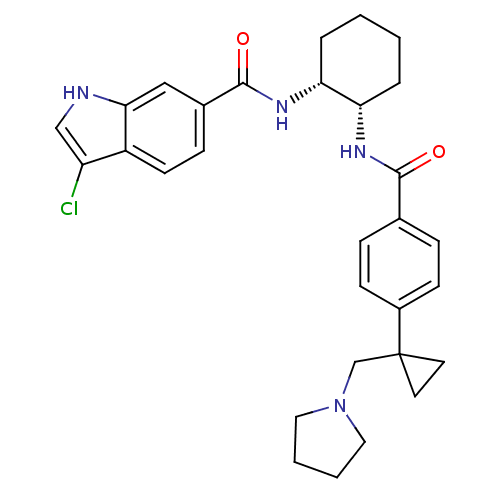
(3-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C30H35ClN4O2/c31-24-18-32-27-17-21(9-12-23(24)27)29(37)34-26-6-2-1-5-25(26)33-28(36)20-7-10-22(11-8-20)30(13-14-30)19-35-15-3-4-16-35/h7-12,17-18,25-26,32H,1-6,13-16,19H2,(H,33,36)(H,34,37)/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216572
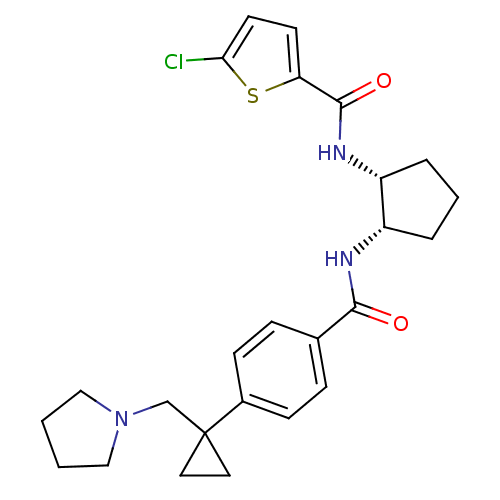
(5-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C25H30ClN3O2S/c26-22-11-10-21(32-22)24(31)28-20-5-3-4-19(20)27-23(30)17-6-8-18(9-7-17)25(12-13-25)16-29-14-1-2-15-29/h6-11,19-20H,1-5,12-16H2,(H,27,30)(H,28,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216556
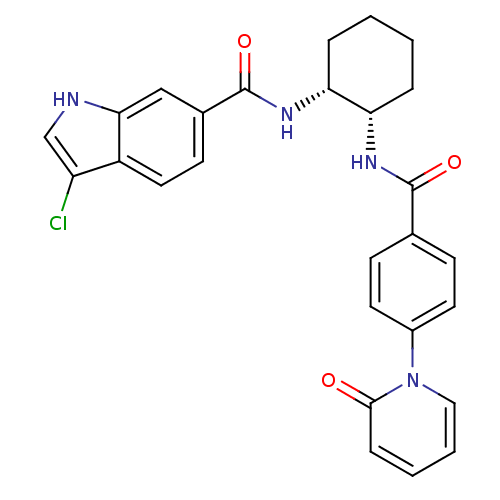
(3-CHLORO-N-((1R,2S) -2-(4-(2-OXOPYRIDIN-1(2H)-YL)B...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216579
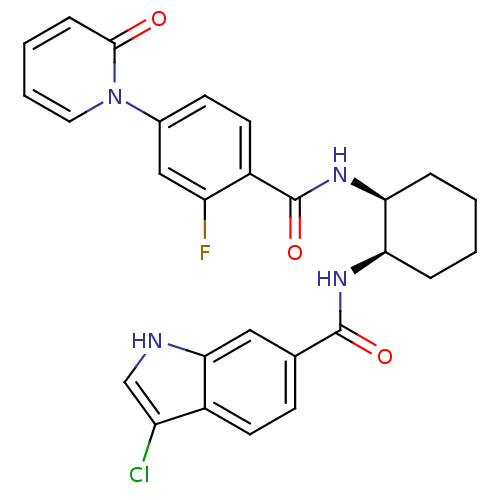
(CHEMBL401024 | cis-3-chloro-N-(2-(2-fluoro-4-(2-ox...)Show SMILES Fc1cc(ccc1C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc2c(Cl)c[nH]c2c1)-n1ccccc1=O Show InChI InChI=1S/C27H24ClFN4O3/c28-20-15-30-24-13-16(8-10-18(20)24)26(35)31-22-5-1-2-6-23(22)32-27(36)19-11-9-17(14-21(19)29)33-12-4-3-7-25(33)34/h3-4,7-15,22-23,30H,1-2,5-6H2,(H,31,35)(H,32,36)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216583
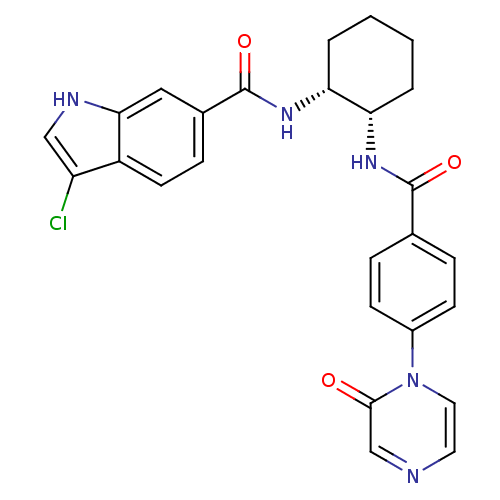
(3-chloro-N-((1R,2S)-2-(4-(2-oxopyrazin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccncc1=O Show InChI InChI=1S/C26H24ClN5O3/c27-20-14-29-23-13-17(7-10-19(20)23)26(35)31-22-4-2-1-3-21(22)30-25(34)16-5-8-18(9-6-16)32-12-11-28-15-24(32)33/h5-15,21-22,29H,1-4H2,(H,30,34)(H,31,35)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216620
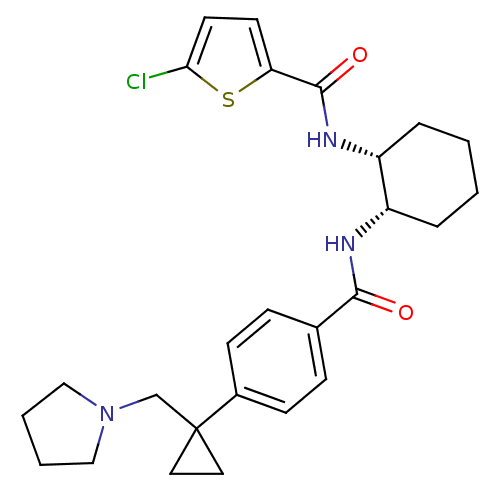
(5-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C26H32ClN3O2S/c27-23-12-11-22(33-23)25(32)29-21-6-2-1-5-20(21)28-24(31)18-7-9-19(10-8-18)26(13-14-26)17-30-15-3-4-16-30/h7-12,20-21H,1-6,13-17H2,(H,28,31)(H,29,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216598
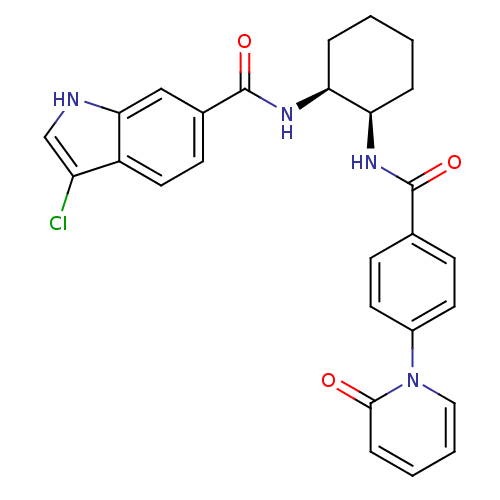
(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216604

(3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H23ClN4O3/c27-20-15-28-23-14-17(9-12-19(20)23)26(34)30-22-5-3-4-21(22)29-25(33)16-7-10-18(11-8-16)31-13-2-1-6-24(31)32/h1-2,6-15,21-22,28H,3-5H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216598

(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216566
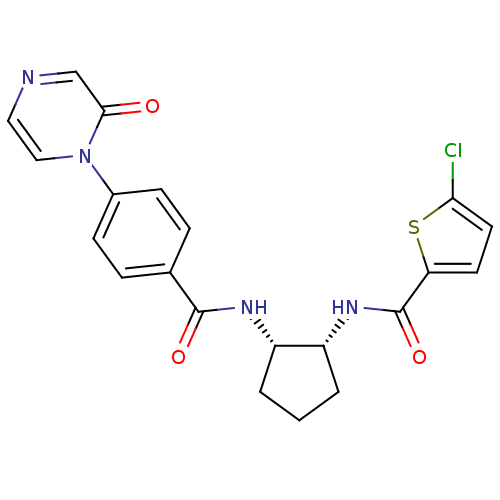
(5-chloro-N-((1R,2S)-2-(4-(2-oxopyrazin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccncc1=O Show InChI InChI=1S/C21H19ClN4O3S/c22-18-9-8-17(30-18)21(29)25-16-3-1-2-15(16)24-20(28)13-4-6-14(7-5-13)26-11-10-23-12-19(26)27/h4-12,15-16H,1-3H2,(H,24,28)(H,25,29)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216576
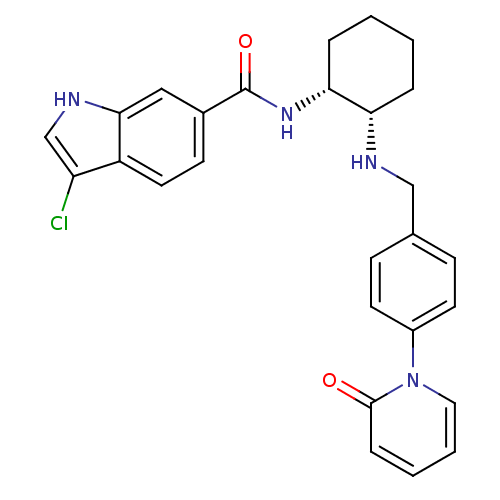
(CHEMBL231940 | cis-N-(2-(4-(2-oxopyridin-1(2H)-yl)...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H27ClN4O2/c28-22-17-30-25-15-19(10-13-21(22)25)27(34)31-24-6-2-1-5-23(24)29-16-18-8-11-20(12-9-18)32-14-4-3-7-26(32)33/h3-4,7-15,17,23-24,29-30H,1-2,5-6,16H2,(H,31,34)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216606
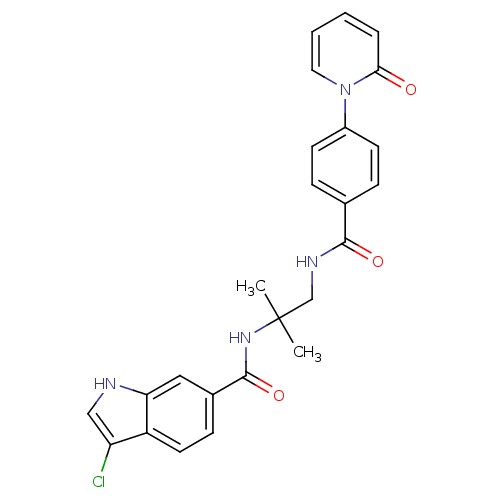
(3-chloro-N-(2-methyl-1-(4-(2-oxopyridin-1(2H)-yl)b...)Show SMILES CC(C)(CNC(=O)c1ccc(cc1)-n1ccccc1=O)NC(=O)c1ccc2c(Cl)c[nH]c2c1 Show InChI InChI=1S/C25H23ClN4O3/c1-25(2,29-24(33)17-8-11-19-20(26)14-27-21(19)13-17)15-28-23(32)16-6-9-18(10-7-16)30-12-4-3-5-22(30)31/h3-14,27H,15H2,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216562
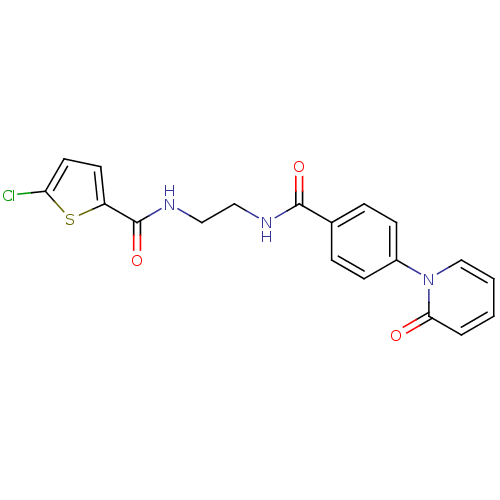
(5-CHLORO-N-(2-(4-(2-OXOPYRIDIN-1(2H)-YL)BENZAMIDO)...)Show SMILES Clc1ccc(s1)C(=O)NCCNC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C19H16ClN3O3S/c20-16-9-8-15(27-16)19(26)22-11-10-21-18(25)13-4-6-14(7-5-13)23-12-2-1-3-17(23)24/h1-9,12H,10-11H2,(H,21,25)(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216557
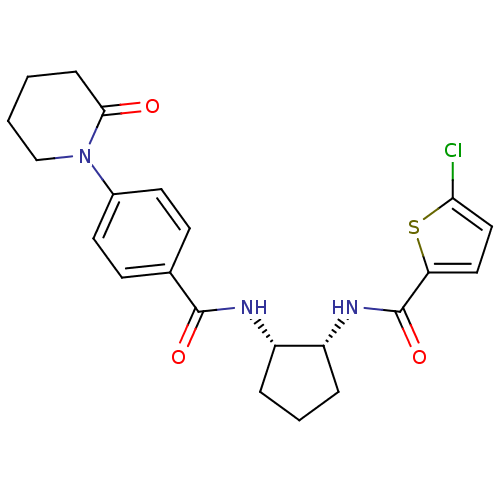
(5-chloro-N-((1R,2S)-2-(4-(2-oxopiperidin-1-yl)benz...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C22H24ClN3O3S/c23-19-12-11-18(30-19)22(29)25-17-5-3-4-16(17)24-21(28)14-7-9-15(10-8-14)26-13-2-1-6-20(26)27/h7-12,16-17H,1-6,13H2,(H,24,28)(H,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216594
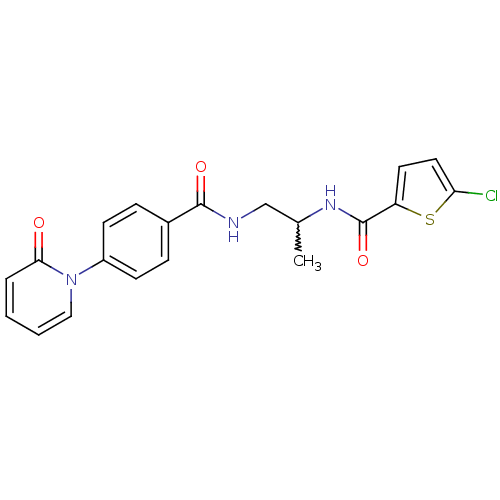
(5-chloro-N-(1-(4-(2-oxopyridin-1(2H)-yl)benzamido)...)Show SMILES CC(CNC(=O)c1ccc(cc1)-n1ccccc1=O)NC(=O)c1ccc(Cl)s1 |w:1.0| Show InChI InChI=1S/C20H18ClN3O3S/c1-13(23-20(27)16-9-10-17(21)28-16)12-22-19(26)14-5-7-15(8-6-14)24-11-3-2-4-18(24)25/h2-11,13H,12H2,1H3,(H,22,26)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216578
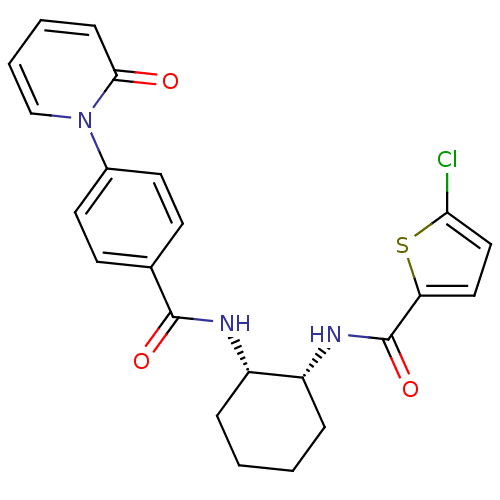
(5-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN3O3S/c24-20-13-12-19(31-20)23(30)26-18-6-2-1-5-17(18)25-22(29)15-8-10-16(11-9-15)27-14-4-3-7-21(27)28/h3-4,7-14,17-18H,1-2,5-6H2,(H,25,29)(H,26,30)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216613
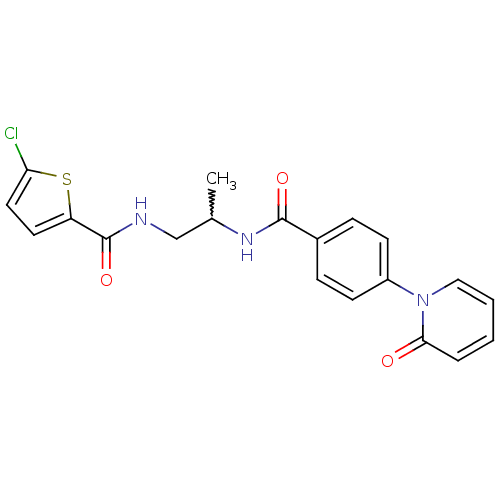
(5-chloro-N-(2-(4-(2-oxopyridin-1(2H)-yl)benzamido)...)Show SMILES CC(CNC(=O)c1ccc(Cl)s1)NC(=O)c1ccc(cc1)-n1ccccc1=O |w:1.0| Show InChI InChI=1S/C20H18ClN3O3S/c1-13(12-22-20(27)16-9-10-17(21)28-16)23-19(26)14-5-7-15(8-6-14)24-11-3-2-4-18(24)25/h2-11,13H,12H2,1H3,(H,22,27)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216622
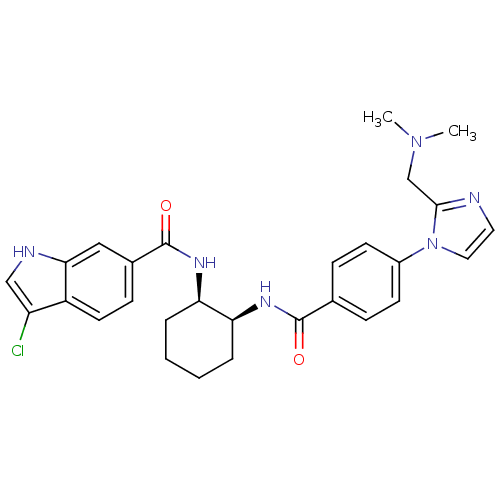
(3-chloro-N-((1R,2S)-2-(4-(2-((dimethylamino)methyl...)Show SMILES CN(C)Cc1nccn1-c1ccc(cc1)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc2c(Cl)c[nH]c2c1 Show InChI InChI=1S/C28H31ClN6O2/c1-34(2)17-26-30-13-14-35(26)20-10-7-18(8-11-20)27(36)32-23-5-3-4-6-24(23)33-28(37)19-9-12-21-22(29)16-31-25(21)15-19/h7-16,23-24,31H,3-6,17H2,1-2H3,(H,32,36)(H,33,37)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216599
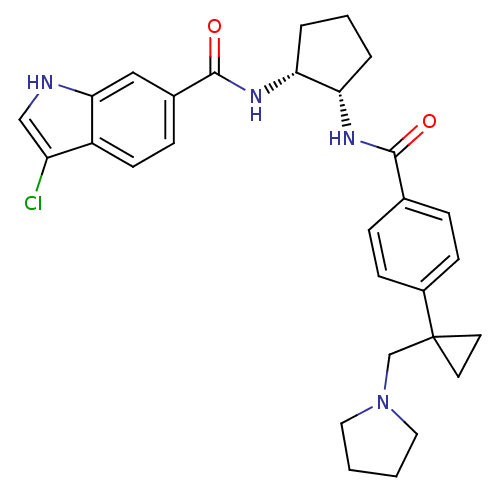
(3-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C29H33ClN4O2/c30-23-17-31-26-16-20(8-11-22(23)26)28(36)33-25-5-3-4-24(25)32-27(35)19-6-9-21(10-7-19)29(12-13-29)18-34-14-1-2-15-34/h6-11,16-17,24-25,31H,1-5,12-15,18H2,(H,32,35)(H,33,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216600
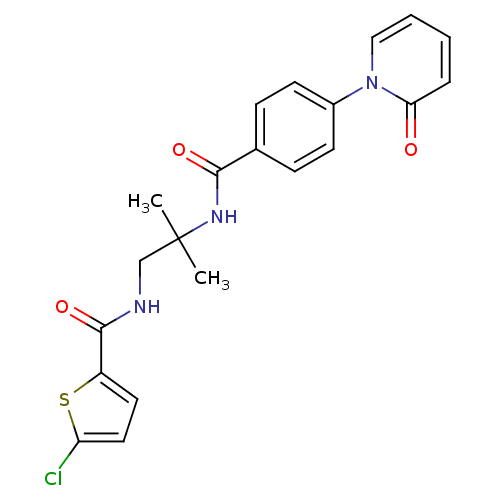
(5-chloro-N-(2-methyl-2-(4-(2-oxopyridin-1(2H)-yl)b...)Show SMILES CC(C)(CNC(=O)c1ccc(Cl)s1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C21H20ClN3O3S/c1-21(2,13-23-20(28)16-10-11-17(22)29-16)24-19(27)14-6-8-15(9-7-14)25-12-4-3-5-18(25)26/h3-12H,13H2,1-2H3,(H,23,28)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216569
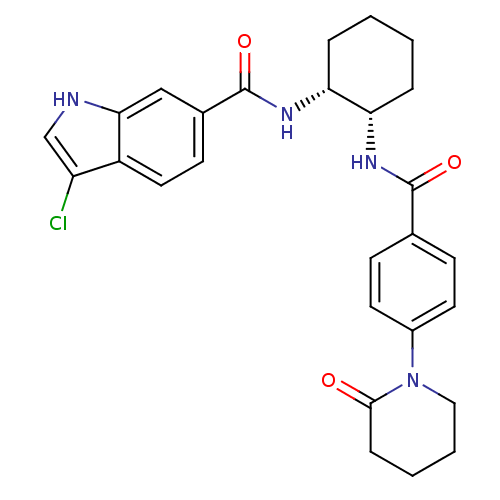
(3-chloro-N-((1R,2S)-2-(4-(2-oxopiperidin-1-yl)benz...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C27H29ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h8-13,15-16,22-23,29H,1-7,14H2,(H,30,34)(H,31,35)/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216618
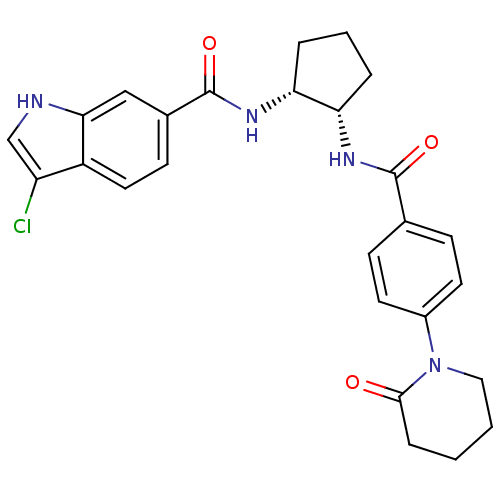
(3-chloro-N-((1R,2S)-2-(4-(2-oxopiperidin-1-yl)benz...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H27ClN4O3/c27-20-15-28-23-14-17(9-12-19(20)23)26(34)30-22-5-3-4-21(22)29-25(33)16-7-10-18(11-8-16)31-13-2-1-6-24(31)32/h7-12,14-15,21-22,28H,1-6,13H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216591
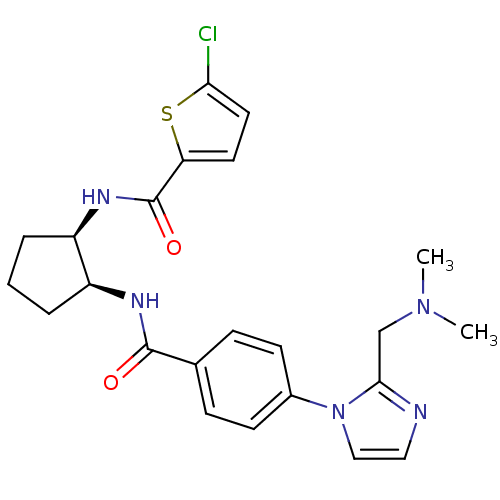
(5-chloro-N-((1R,2S)-2-(4-(2-((dimethylamino)methyl...)Show SMILES CN(C)Cc1nccn1-c1ccc(cc1)C(=O)N[C@H]1CCC[C@H]1NC(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C23H26ClN5O2S/c1-28(2)14-21-25-12-13-29(21)16-8-6-15(7-9-16)22(30)26-17-4-3-5-18(17)27-23(31)19-10-11-20(24)32-19/h6-13,17-18H,3-5,14H2,1-2H3,(H,26,30)(H,27,31)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216597
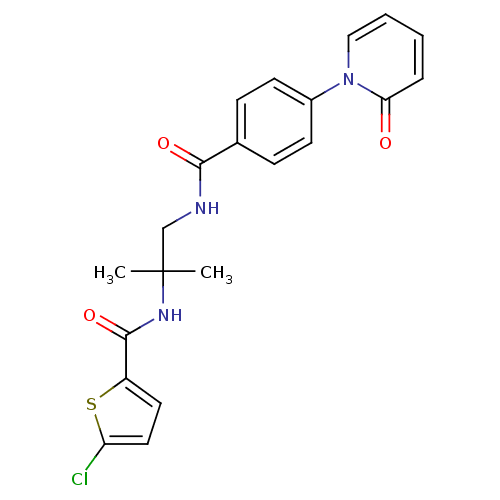
(5-chloro-N-(2-methyl-1-(4-(2-oxopyridin-1(2H)-yl)b...)Show SMILES CC(C)(CNC(=O)c1ccc(cc1)-n1ccccc1=O)NC(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C21H20ClN3O3S/c1-21(2,24-20(28)16-10-11-17(22)29-16)13-23-19(27)14-6-8-15(9-7-14)25-12-4-3-5-18(25)26/h3-12H,13H2,1-2H3,(H,23,27)(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216571
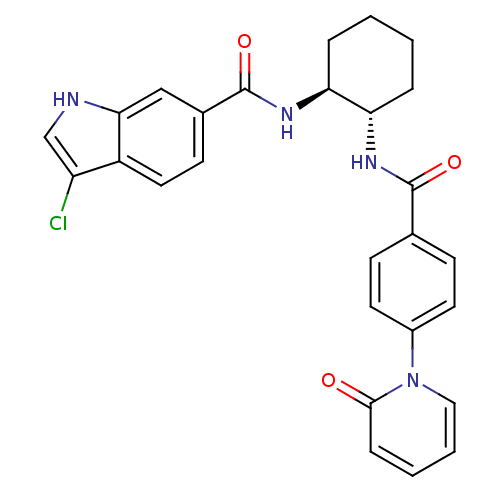
(CHEMBL231787 | trans-(+-)-3-chloro-N-(2-(4-(2-oxop...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216582
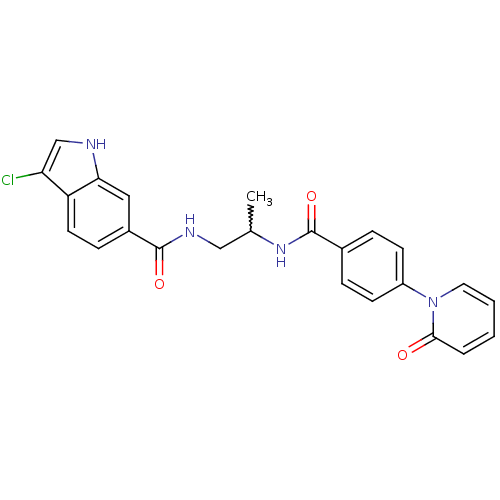
(3-chloro-N-(2-(4-(2-oxopyridin-1(2H)-yl)benzamido)...)Show SMILES CC(CNC(=O)c1ccc2c(Cl)c[nH]c2c1)NC(=O)c1ccc(cc1)-n1ccccc1=O |w:1.0| Show InChI InChI=1S/C24H21ClN4O3/c1-15(13-27-23(31)17-7-10-19-20(25)14-26-21(19)12-17)28-24(32)16-5-8-18(9-6-16)29-11-3-2-4-22(29)30/h2-12,14-15,26H,13H2,1H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216596
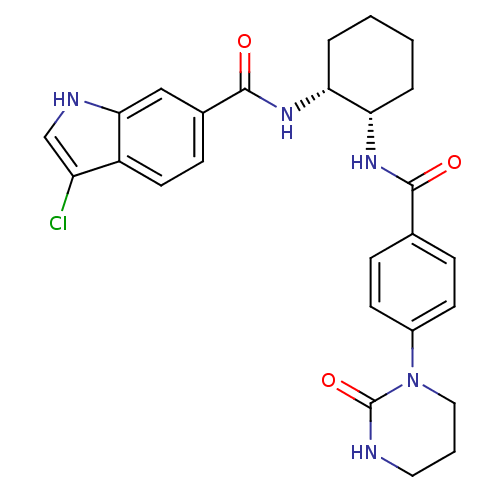
(3-chloro-N-((1R,2S)-2-(4-(2-oxo-tetrahydropyrimidi...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCNC1=O Show InChI InChI=1S/C26H28ClN5O3/c27-20-15-29-23-14-17(8-11-19(20)23)25(34)31-22-5-2-1-4-21(22)30-24(33)16-6-9-18(10-7-16)32-13-3-12-28-26(32)35/h6-11,14-15,21-22,29H,1-5,12-13H2,(H,28,35)(H,30,33)(H,31,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216586
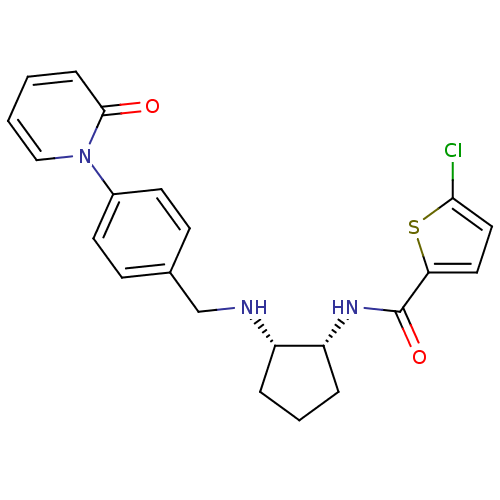
(CHEMBL233173 | cis-N-(2-(4-(2-oxopyridin-1(2H)-yl)...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NCc1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H22ClN3O2S/c23-20-12-11-19(29-20)22(28)25-18-5-3-4-17(18)24-14-15-7-9-16(10-8-15)26-13-2-1-6-21(26)27/h1-2,6-13,17-18,24H,3-5,14H2,(H,25,28)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216616
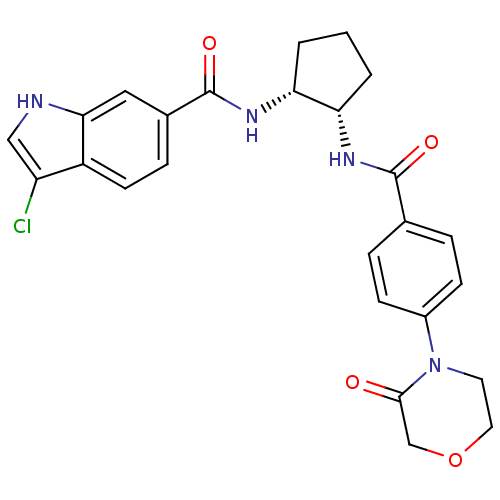
(3-chloro-N-((1R,2S)-2-(4-(3-oxomorpholino)benzamid...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)N1CCOCC1=O Show InChI InChI=1S/C25H25ClN4O4/c26-19-13-27-22-12-16(6-9-18(19)22)25(33)29-21-3-1-2-20(21)28-24(32)15-4-7-17(8-5-15)30-10-11-34-14-23(30)31/h4-9,12-13,20-21,27H,1-3,10-11,14H2,(H,28,32)(H,29,33)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
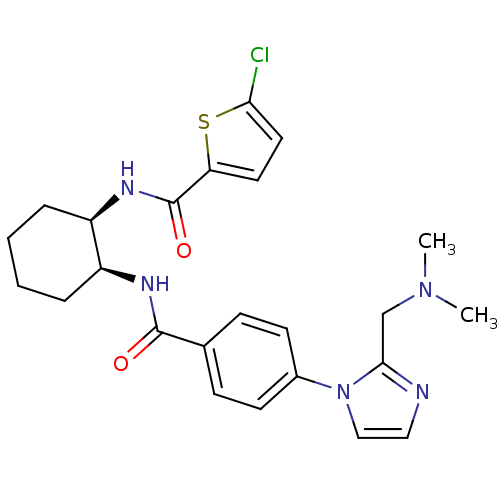
(Homo sapiens (Human)) | BDBM50216607
(5-chloro-N-((1R,2S)-2-(4-(2-((dimethylamino)methyl...)Show SMILES CN(C)Cc1nccn1-c1ccc(cc1)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C24H28ClN5O2S/c1-29(2)15-22-26-13-14-30(22)17-9-7-16(8-10-17)23(31)27-18-5-3-4-6-19(18)28-24(32)20-11-12-21(25)33-20/h7-14,18-19H,3-6,15H2,1-2H3,(H,27,31)(H,28,32)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
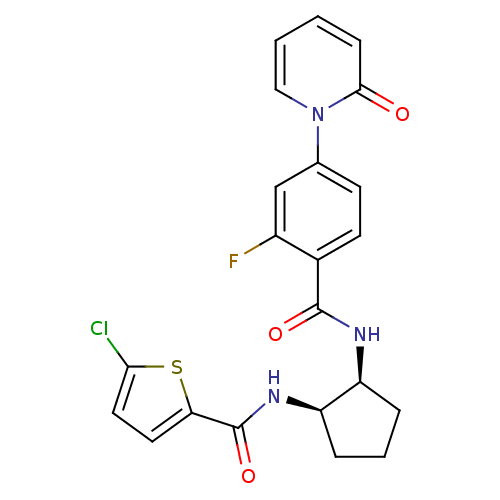
(Homo sapiens (Human)) | BDBM50216614
(CHEMBL393521 | cis-5-chloro-N-(2-(2-fluoro-4-(2-ox...)Show SMILES Fc1cc(ccc1C(=O)N[C@H]1CCC[C@H]1NC(=O)c1ccc(Cl)s1)-n1ccccc1=O Show InChI InChI=1S/C22H19ClFN3O3S/c23-19-10-9-18(31-19)22(30)26-17-5-3-4-16(17)25-21(29)14-8-7-13(12-15(14)24)27-11-2-1-6-20(27)28/h1-2,6-12,16-17H,3-5H2,(H,25,29)(H,26,30)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
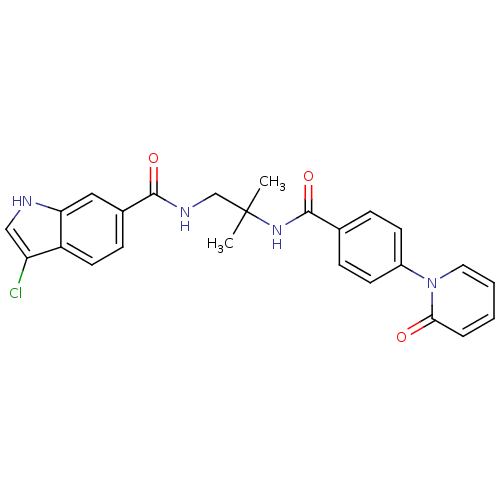
(Homo sapiens (Human)) | BDBM50216580
(3-chloro-N-(2-methyl-2-(4-(2-oxopyridin-1(2H)-yl)b...)Show SMILES CC(C)(CNC(=O)c1ccc2c(Cl)c[nH]c2c1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H23ClN4O3/c1-25(2,15-28-23(32)17-8-11-19-20(26)14-27-21(19)13-17)29-24(33)16-6-9-18(10-7-16)30-12-4-3-5-22(30)31/h3-14,27H,15H2,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
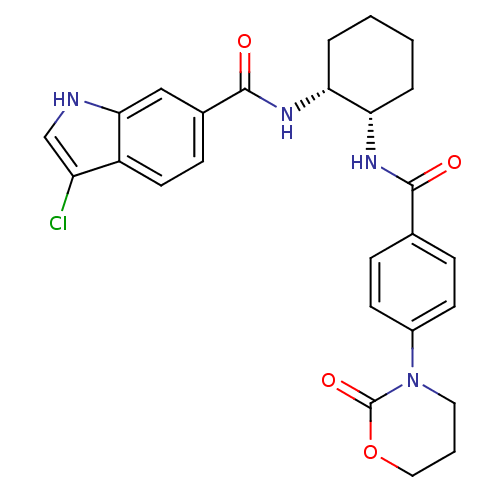
(Homo sapiens (Human)) | BDBM50216612
(3-chloro-N-((1R,2S)-2-(4-(2-oxo-1,3-oxazinan-3-yl)...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCOC1=O Show InChI InChI=1S/C26H27ClN4O4/c27-20-15-28-23-14-17(8-11-19(20)23)25(33)30-22-5-2-1-4-21(22)29-24(32)16-6-9-18(10-7-16)31-12-3-13-35-26(31)34/h6-11,14-15,21-22,28H,1-5,12-13H2,(H,29,32)(H,30,33)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216593
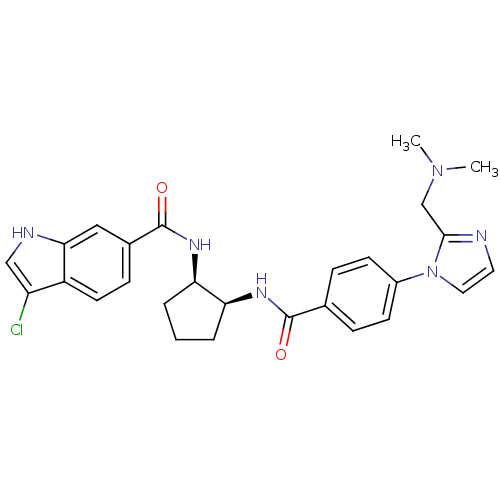
(3-chloro-N-((1R,2S)-2-(4-(2-((dimethylamino)methyl...)Show SMILES CN(C)Cc1nccn1-c1ccc(cc1)C(=O)N[C@H]1CCC[C@H]1NC(=O)c1ccc2c(Cl)c[nH]c2c1 Show InChI InChI=1S/C27H29ClN6O2/c1-33(2)16-25-29-12-13-34(25)19-9-6-17(7-10-19)26(35)31-22-4-3-5-23(22)32-27(36)18-8-11-20-21(28)15-30-24(20)14-18/h6-15,22-23,30H,3-5,16H2,1-2H3,(H,31,35)(H,32,36)/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216553
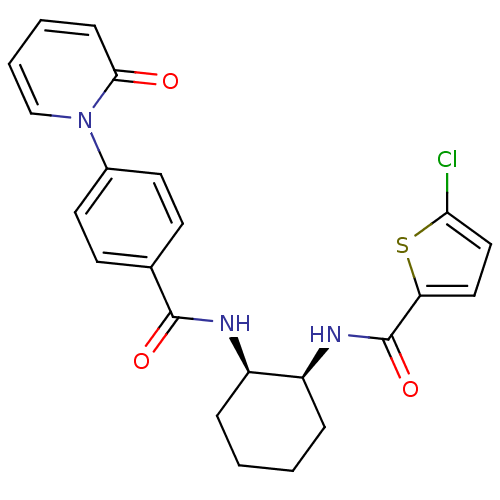
(CHEMBL391527 | cis-(+/-)-5-chloro-N-(2-(4-(2-oxopy...)Show SMILES Clc1ccc(s1)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN3O3S/c24-20-13-12-19(31-20)23(30)26-18-6-2-1-5-17(18)25-22(29)15-8-10-16(11-9-15)27-14-4-3-7-21(27)28/h3-4,7-14,17-18H,1-2,5-6H2,(H,25,29)(H,26,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216573
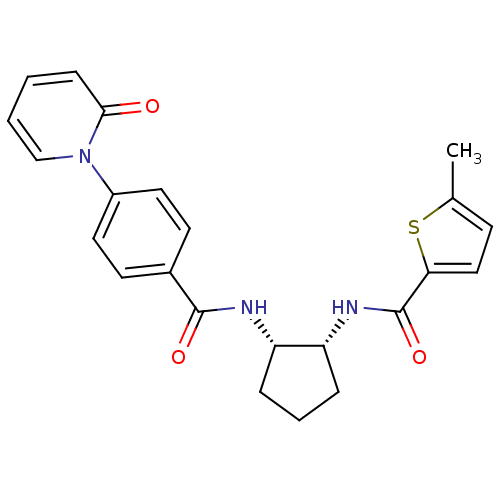
(5-methyl-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Cc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H23N3O3S/c1-15-8-13-20(30-15)23(29)25-19-6-4-5-18(19)24-22(28)16-9-11-17(12-10-16)26-14-3-2-7-21(26)27/h2-3,7-14,18-19H,4-6H2,1H3,(H,24,28)(H,25,29)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216617
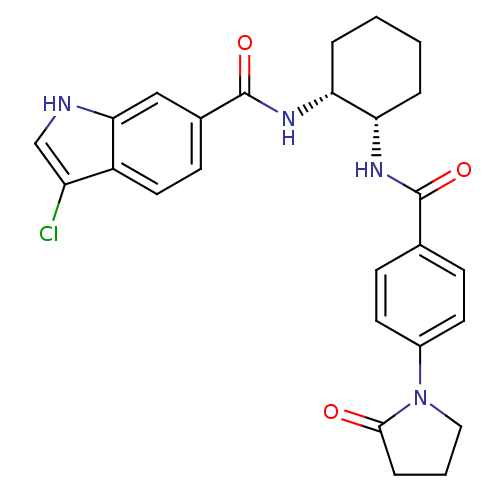
(3-chloro-N-((1R,2S)-2-(4-(2-oxopyrrolidin-1-yl)ben...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C26H27ClN4O3/c27-20-15-28-23-14-17(9-12-19(20)23)26(34)30-22-5-2-1-4-21(22)29-25(33)16-7-10-18(11-8-16)31-13-3-6-24(31)32/h7-12,14-15,21-22,28H,1-6,13H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216592
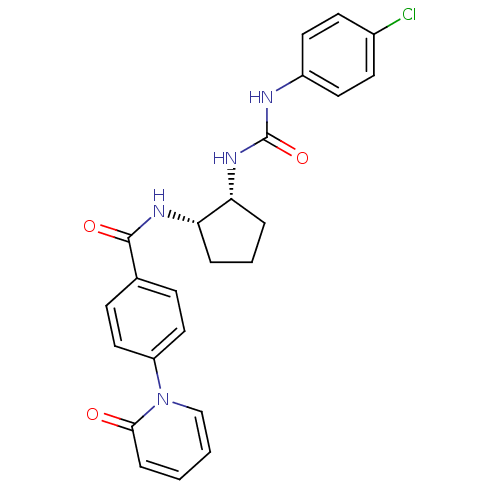
(CHEMBL440969 | cis-1-(4-chlorophenyl)-3-(2-(4-(2-o...)Show SMILES Clc1ccc(NC(=O)N[C@@H]2CCC[C@@H]2NC(=O)c2ccc(cc2)-n2ccccc2=O)cc1 Show InChI InChI=1S/C24H23ClN4O3/c25-17-9-11-18(12-10-17)26-24(32)28-21-5-3-4-20(21)27-23(31)16-7-13-19(14-8-16)29-15-2-1-6-22(29)30/h1-2,6-15,20-21H,3-5H2,(H,27,31)(H2,26,28,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216589
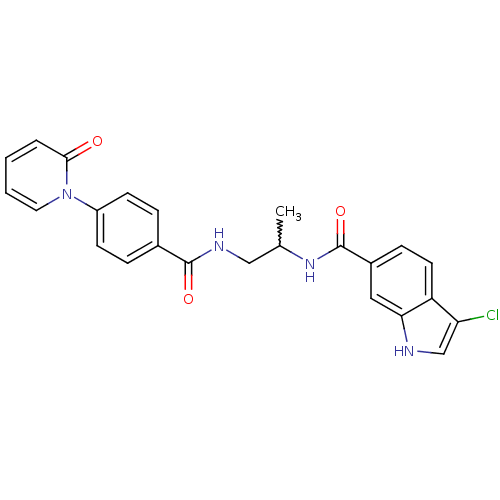
(3-chloro-N-(1-(4-(2-oxopyridin-1(2H)-yl)benzamido)...)Show SMILES CC(CNC(=O)c1ccc(cc1)-n1ccccc1=O)NC(=O)c1ccc2c(Cl)c[nH]c2c1 |w:1.0| Show InChI InChI=1S/C24H21ClN4O3/c1-15(28-24(32)17-7-10-19-20(25)14-26-21(19)12-17)13-27-23(31)16-5-8-18(9-6-16)29-11-3-2-4-22(29)30/h2-12,14-15,26H,13H2,1H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216561
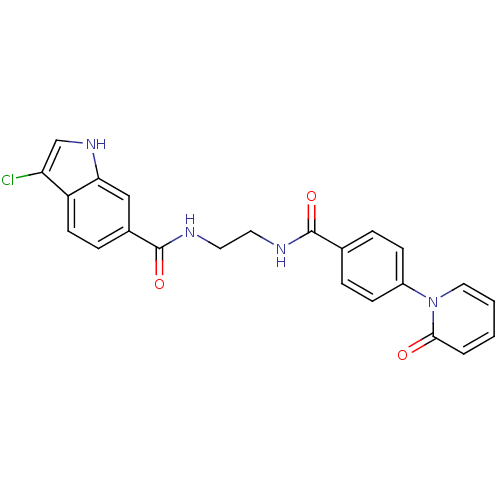
(3-chloro-N-(2-(4-(2-oxopyridin-1(2H)-yl)benzamido)...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)NCCNC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H19ClN4O3/c24-19-14-27-20-13-16(6-9-18(19)20)23(31)26-11-10-25-22(30)15-4-7-17(8-5-15)28-12-2-1-3-21(28)29/h1-9,12-14,27H,10-11H2,(H,25,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216603
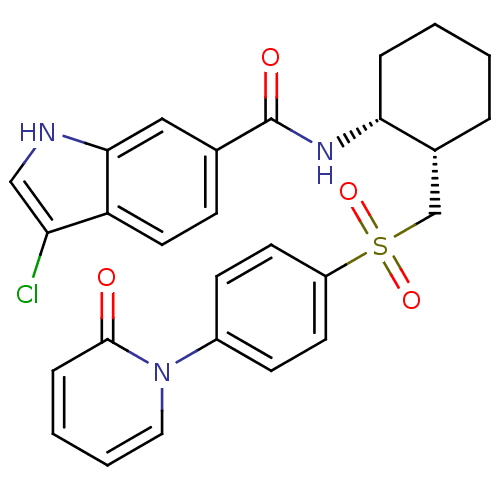
(CHEMBL231726 | cis-3-chloro-N-(2-((4-(2-oxopyridin...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1CS(=O)(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H26ClN3O4S/c28-23-16-29-25-15-18(8-13-22(23)25)27(33)30-24-6-2-1-5-19(24)17-36(34,35)21-11-9-20(10-12-21)31-14-4-3-7-26(31)32/h3-4,7-16,19,24,29H,1-2,5-6,17H2,(H,30,33)/t19-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
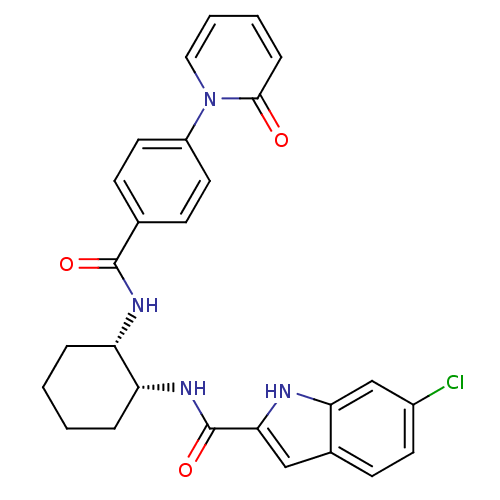
(Homo sapiens (Human)) | BDBM50216575
(6-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc2cc([nH]c2c1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-19-11-8-18-15-24(29-23(18)16-19)27(35)31-22-6-2-1-5-21(22)30-26(34)17-9-12-20(13-10-17)32-14-4-3-7-25(32)33/h3-4,7-16,21-22,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
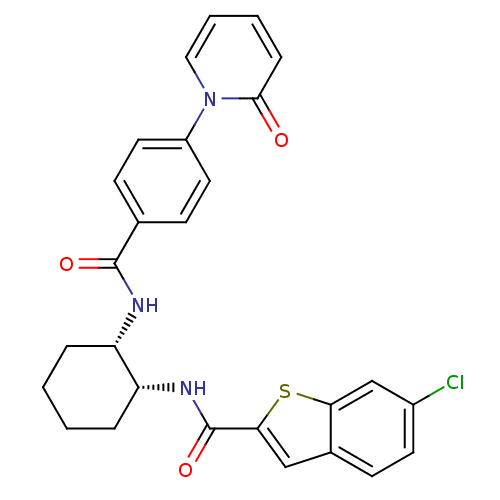
(Homo sapiens (Human)) | BDBM50216601
(6-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc2cc(sc2c1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H24ClN3O3S/c28-19-11-8-18-15-24(35-23(18)16-19)27(34)30-22-6-2-1-5-21(22)29-26(33)17-9-12-20(13-10-17)31-14-4-3-7-25(31)32/h3-4,7-16,21-22H,1-2,5-6H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
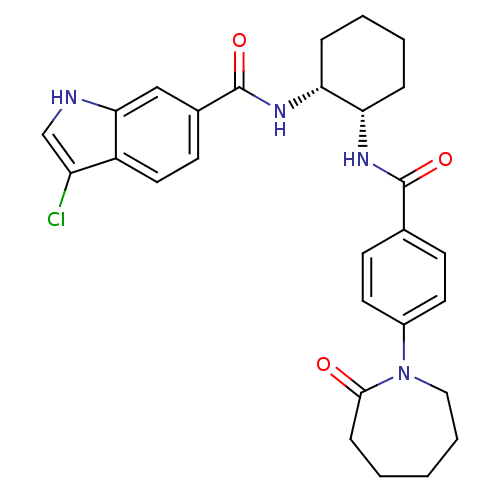
(Homo sapiens (Human)) | BDBM50216602
(3-chloro-N-((1R,2S)-2-(4-(2-oxoazepan-1-yl)benzami...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCCCC1=O Show InChI InChI=1S/C28H31ClN4O3/c29-22-17-30-25-16-19(11-14-21(22)25)28(36)32-24-7-4-3-6-23(24)31-27(35)18-9-12-20(13-10-18)33-15-5-1-2-8-26(33)34/h9-14,16-17,23-24,30H,1-8,15H2,(H,31,35)(H,32,36)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
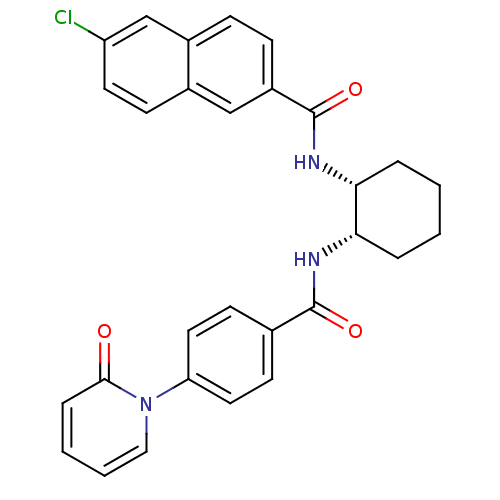
(Homo sapiens (Human)) | BDBM50216588
(6-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc2cc(ccc2c1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C29H26ClN3O3/c30-23-13-10-20-17-22(9-8-21(20)18-23)29(36)32-26-6-2-1-5-25(26)31-28(35)19-11-14-24(15-12-19)33-16-4-3-7-27(33)34/h3-4,7-18,25-26H,1-2,5-6H2,(H,31,35)(H,32,36)/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216609
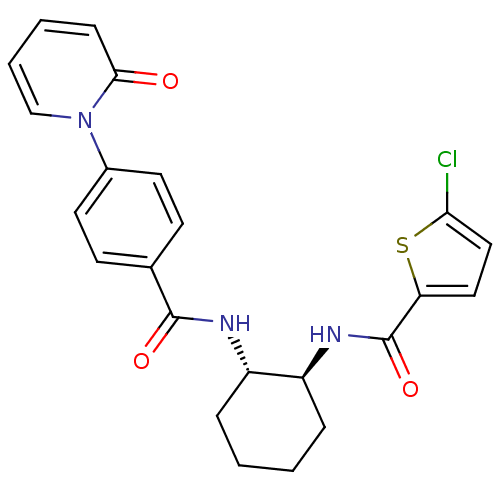
(CHEMBL233806 | trans-(+/-)-5-chloro-N-(2-(4-(2-oxo...)Show SMILES Clc1ccc(s1)C(=O)N[C@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN3O3S/c24-20-13-12-19(31-20)23(30)26-18-6-2-1-5-17(18)25-22(29)15-8-10-16(11-9-15)27-14-4-3-7-21(27)28/h3-4,7-14,17-18H,1-2,5-6H2,(H,25,29)(H,26,30)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216565
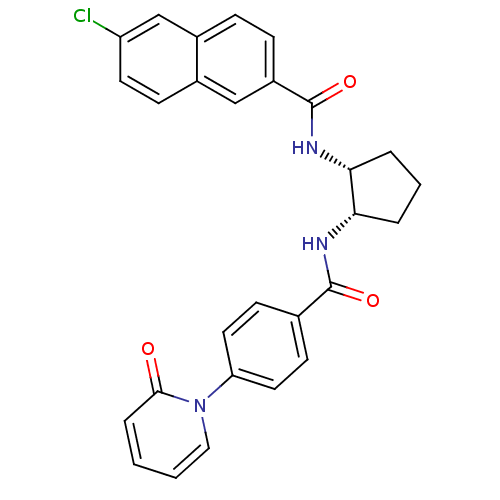
(6-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc2cc(ccc2c1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C28H24ClN3O3/c29-22-12-9-19-16-21(8-7-20(19)17-22)28(35)31-25-5-3-4-24(25)30-27(34)18-10-13-23(14-11-18)32-15-2-1-6-26(32)33/h1-2,6-17,24-25H,3-5H2,(H,30,34)(H,31,35)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data