 Found 15 hits Enz. Inhib. hit(s) with all data for entry = 50038057
Found 15 hits Enz. Inhib. hit(s) with all data for entry = 50038057 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM50213693
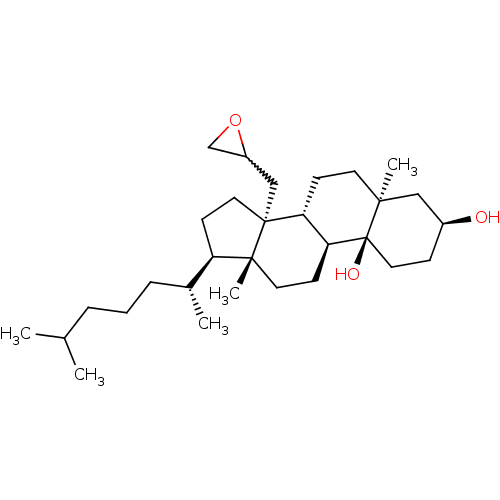
((3S,5S,8R,9S,10S,13R,14R,17R)-5,13-dimethyl-17-((R...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@@]2(CC3CO3)[C@@H]3CC[C@@]4(C)C[C@@H](O)CC[C@]4(O)[C@H]3CC[C@]12C |w:13.12| Show InChI InChI=1S/C30H52O3/c1-20(2)7-6-8-21(3)24-12-15-29(18-23-19-33-23)25-10-13-27(4)17-22(31)9-16-30(27,32)26(25)11-14-28(24,29)5/h20-26,31-32H,6-19H2,1-5H3/t21-,22+,23?,24-,25-,26+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of rat liver LDM |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
ATP-citrate synthase
(Homo sapiens (Human)) | BDBM50066683
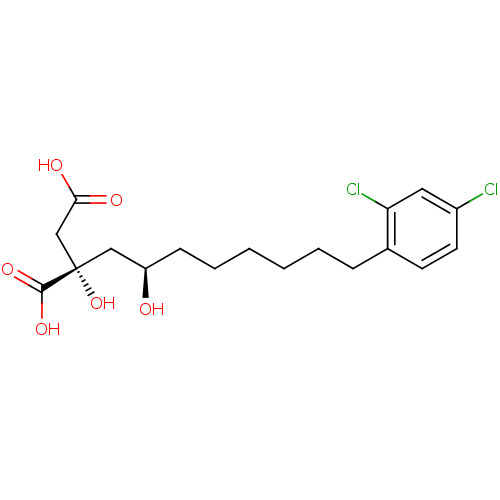
((S)-2-((R)-8-(2,4-dichlorophenyl)-2-hydroxyoctyl)-...)Show SMILES O[C@H](CCCCCCc1ccc(Cl)cc1Cl)C[C@](O)(CC(O)=O)C(O)=O Show InChI InChI=1S/C18H24Cl2O6/c19-13-8-7-12(15(20)9-13)5-3-1-2-4-6-14(21)10-18(26,17(24)25)11-16(22)23/h7-9,14,21,26H,1-6,10-11H2,(H,22,23)(H,24,25)/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human ATP citrate lyase |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Candida albicans (strain SC5314 / ATCC MYA-2876) (...) | BDBM50055641
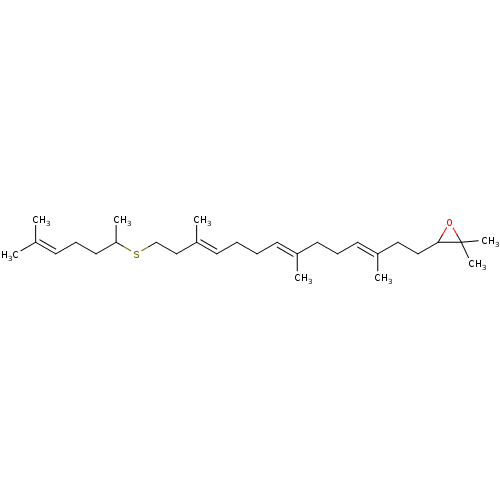
(2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#16]-[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]1-[#8]C1([#6])[#6] Show InChI InChI=1S/C29H50OS/c1-23(2)13-11-18-27(6)31-22-21-26(5)15-10-9-14-24(3)16-12-17-25(4)19-20-28-29(7,8)30-28/h13-15,17,27-28H,9-12,16,18-22H2,1-8H3/b24-14+,25-17+,26-15+ | KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Candida albicans OSC |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50094519
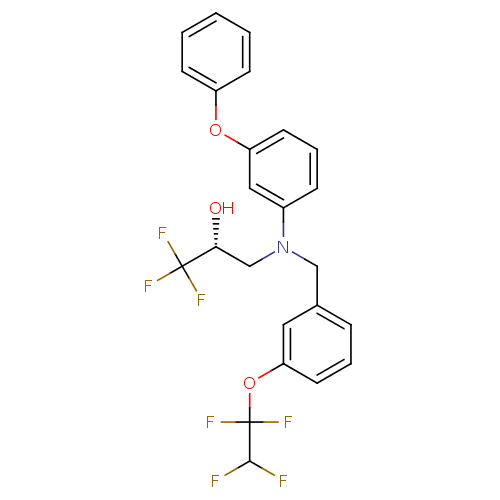
((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...)Show SMILES O[C@H](CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C24H20F7NO3/c25-22(26)24(30,31)35-20-11-4-6-16(12-20)14-32(15-21(33)23(27,28)29)17-7-5-10-19(13-17)34-18-8-2-1-3-9-18/h1-13,21-22,33H,14-15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of CETP assessed as transfer of [3H]cholesterol esters from HDL to LDL |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Rattus norvegicus) | BDBM50213696
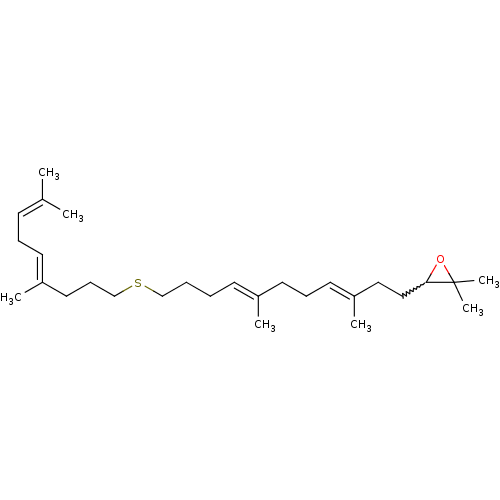
(3-((3E,7E)-11-((E)-4,8-dimethylnona-4,7-dienylthio...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]-[#16]-[#6]-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]1-[#8]C1([#6])[#6] |w:25.24| Show InChI InChI=1S/C28H48OS/c1-23(2)13-10-15-25(4)18-12-22-30-21-9-8-14-24(3)16-11-17-26(5)19-20-27-28(6,7)29-27/h13-15,17,27H,8-12,16,18-22H2,1-7H3/b24-14+,25-15+,26-17+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of rat OSC |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Rattus norvegicus) | BDBM50055641

(2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#16]-[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]1-[#8]C1([#6])[#6] Show InChI InChI=1S/C29H50OS/c1-23(2)13-11-18-27(6)31-22-21-26(5)15-10-9-14-24(3)16-12-17-25(4)19-20-28-29(7,8)30-28/h13-15,17,27-28H,9-12,16,18-22H2,1-8H3/b24-14+,25-17+,26-15+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of rat OSC |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Rattus norvegicus) | BDBM50213692
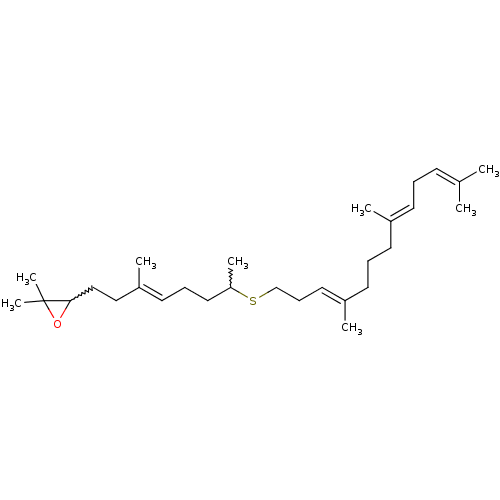
(2,2-dimethyl-3-((E)-3-methyl-7-((3E,8E)-4,8,12-tri...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]1-[#8]C1([#6])[#6])-[#16]-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]-[#6]\[#6](-[#6])=[#6]\[#6]\[#6]=[#6](\[#6])-[#6] |w:9.8,1.0| Show InChI InChI=1S/C29H50OS/c1-23(2)13-9-14-24(3)15-10-16-25(4)18-12-22-31-27(6)19-11-17-26(5)20-21-28-29(7,8)30-28/h13-14,17-18,27-28H,9-12,15-16,19-22H2,1-8H3/b24-14+,25-18+,26-17+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of rat OSC |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50094519

((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...)Show SMILES O[C@H](CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C24H20F7NO3/c25-22(26)24(30,31)35-20-11-4-6-16(12-20)14-32(15-21(33)23(27,28)29)17-7-5-10-19(13-17)34-18-8-2-1-3-9-18/h1-13,21-22,33H,14-15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of human CETP assessed as transfer of [3H]cholesterol esters from HDL to LDL in humna plasma |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Rattus norvegicus) | BDBM50213691
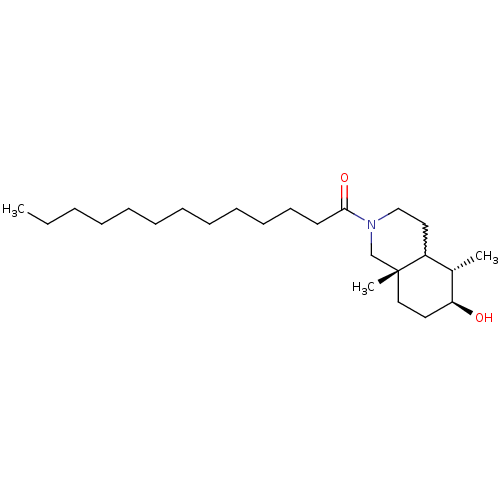
(1-((5S,6S,8aS)-6-hydroxy-5,8a-dimethyl-octahydrois...)Show SMILES CCCCCCCCCCCCC(=O)N1CCC2[C@H](C)[C@@H](O)CC[C@]2(C)C1 |w:17.16| Show InChI InChI=1S/C24H45NO2/c1-4-5-6-7-8-9-10-11-12-13-14-23(27)25-18-16-21-20(2)22(26)15-17-24(21,3)19-25/h20-22,26H,4-19H2,1-3H3/t20-,21?,22-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of rat OSC |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50213683

(CHEMBL218693 | carnosol)Show SMILES CC(C)c1cc2[C@@H]3C[C@H]4C(C)(C)CCC[C@]4(C(=O)O3)c2c(O)c1O |r| Show InChI InChI=1S/C20H26O4/c1-10(2)11-8-12-13-9-14-19(3,4)6-5-7-20(14,18(23)24-13)15(12)17(22)16(11)21/h8,10,13-14,21-22H,5-7,9H2,1-4H3/t13-,14-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pancreatic lipase |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50213689
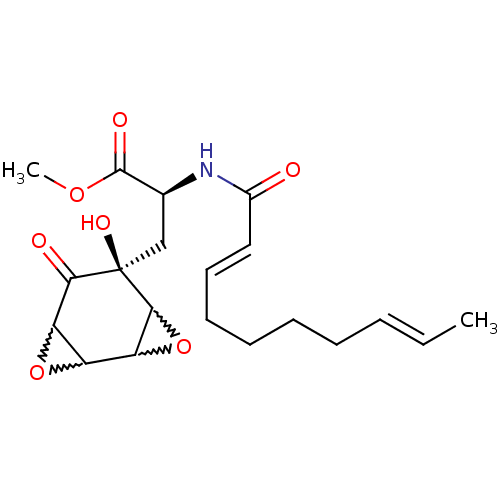
((S)-2-((2E,8E)-deca-2,8-dienoylamino)-3-((S)-5-hyd...)Show SMILES COC(=O)[C@H](C[C@]1(O)C2OC2C2OC2C1=O)NC(=O)\C=C\CCCC\C=C\C |w:10.9,8.8,13.13,11.12| Show InChI InChI=1S/C20H27NO7/c1-3-4-5-6-7-8-9-10-13(22)21-12(19(24)26-2)11-20(25)17(23)15-14(27-15)16-18(20)28-16/h3-4,9-10,12,14-16,18,25H,5-8,11H2,1-2H3,(H,21,22)/b4-3+,10-9+/t12-,14?,15?,16?,18?,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pancreatic lipase |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50371232
(CARNOSIC ACID)Show SMILES CC(C)c1cc2CC[C@H]3C(C)(C)CCC[C@]3(C(O)=O)c2c(O)c1O |r| Show InChI InChI=1S/C20H28O4/c1-11(2)13-10-12-6-7-14-19(3,4)8-5-9-20(14,18(23)24)15(12)17(22)16(13)21/h10-11,14,21-22H,5-9H2,1-4H3,(H,23,24)/t14-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pancreatic lipase |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM50213698
((1R,3aS,3bR,5aS,7S,9aS,9bS,11aR,E)-3,10-dihydroxy-...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC(N=O)[C@@]2(C)[C@@H]3CC[C@@]4(C)C[C@@H](O)CC[C@]4(O)[C@H]3CC[C@]12C Show InChI InChI=1S/C28H49NO3/c1-18(2)8-7-9-19(3)23-16-24(29-32)27(6)21-11-13-25(4)17-20(30)10-15-28(25,31)22(21)12-14-26(23,27)5/h18-24,30-31H,7-17H2,1-6H3/t19-,20+,21-,22+,23-,24?,25+,26-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of rat liver LDM |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50213686
(2-((2E,8E)-deca-2,8-dienoylamino)-3-(5-hydroxy-6-o...)Show SMILES COC(=O)C(CC1(O)C2OC2C2OC2C1=O)NC(=O)\C=C\CCCC\C=C\C |w:4.18,10.9,8.8,6.6,13.13,11.12| Show InChI InChI=1S/C20H27NO7/c1-3-4-5-6-7-8-9-10-13(22)21-12(19(24)26-2)11-20(25)17(23)15-14(27-15)16-18(20)28-16/h3-4,9-10,12,14-16,18,25H,5-8,11H2,1-2H3,(H,21,22)/b4-3+,10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pancreatic lipase |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50213694

((R)-2-((2E,8E)-deca-2,8-dienoylamino)-3-((R)-5-hyd...)Show SMILES COC(=O)[C@@H](C[C@@]1(O)C2OC2C2OC2C1=O)NC(=O)\C=C\CCCC\C=C\C |w:10.9,8.8,13.13,11.12| Show InChI InChI=1S/C20H27NO7/c1-3-4-5-6-7-8-9-10-13(22)21-12(19(24)26-2)11-20(25)17(23)15-14(27-15)16-18(20)28-16/h3-4,9-10,12,14-16,18,25H,5-8,11H2,1-2H3,(H,21,22)/b4-3+,10-9+/t12-,14?,15?,16?,18?,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pancreatic lipase |
Bioorg Med Chem 15: 4674-99 (2007)
Article DOI: 10.1016/j.bmc.2007.04.031
BindingDB Entry DOI: 10.7270/Q24J0FZ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data