 Found 114 hits Enz. Inhib. hit(s) with all data for entry = 50038578
Found 114 hits Enz. Inhib. hit(s) with all data for entry = 50038578 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271367
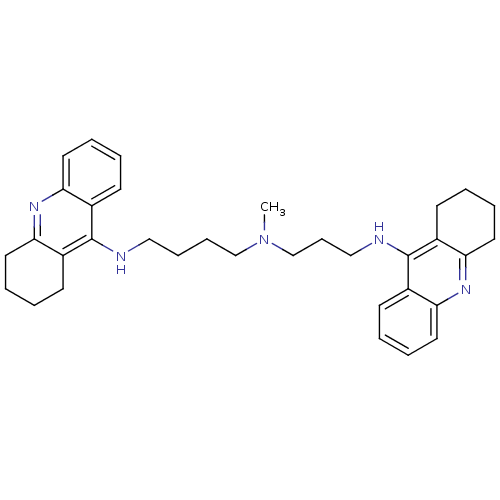
(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50271556
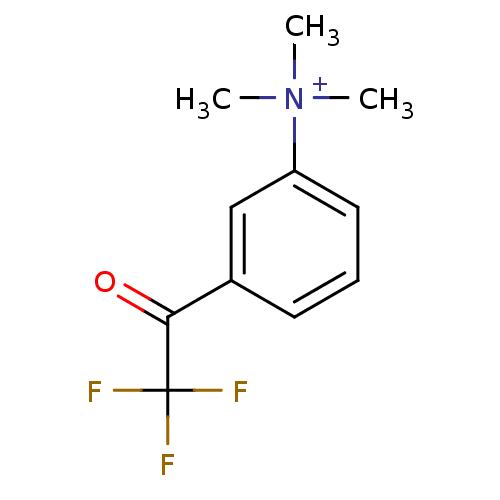
(CHEMBL525622 | N,N,N-trimethyl-3-(2,2,2-trifluoroa...)Show InChI InChI=1S/C11H13F3NO/c1-15(2,3)9-6-4-5-8(7-9)10(16)11(12,13)14/h4-7H,1-3H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50149201
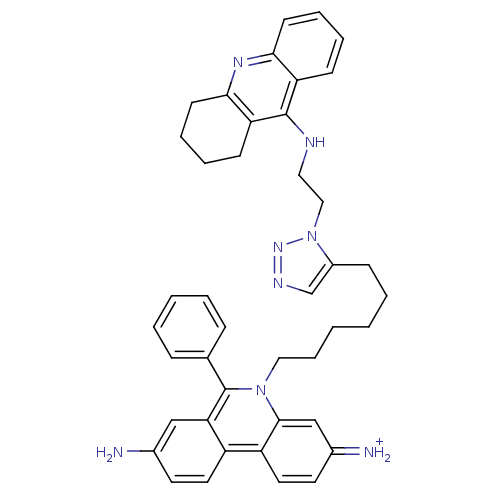
(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271469
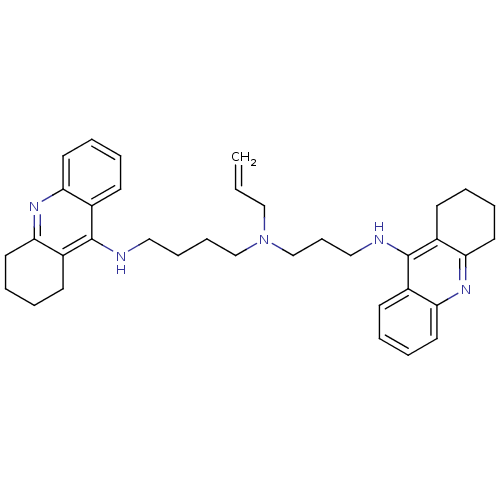
(CHEMBL507174 | N-Allyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES C=CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H45N5/c1-2-24-41(26-13-23-38-36-29-16-5-9-20-33(29)40-34-21-10-6-17-30(34)36)25-12-11-22-37-35-27-14-3-7-18-31(27)39-32-19-8-4-15-28(32)35/h2-3,5,7,9,14,16,18,20H,1,4,6,8,10-13,15,17,19,21-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271470
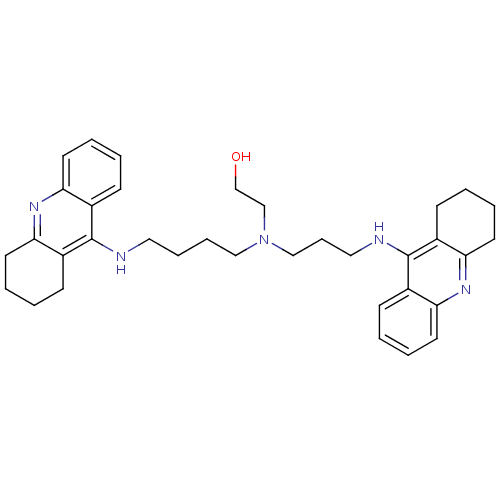
(CHEMBL499224 | N-(2-Hydroxyethyl)-N-(1,2,3,4-tetra...)Show SMILES OCCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5O/c41-25-24-40(23-11-21-37-35-28-14-3-7-18-32(28)39-33-19-8-4-15-29(33)35)22-10-9-20-36-34-26-12-1-5-16-30(26)38-31-17-6-2-13-27(31)34/h1,3,5,7,12,14,16,18,41H,2,4,6,8-11,13,15,17,19-25H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50369748
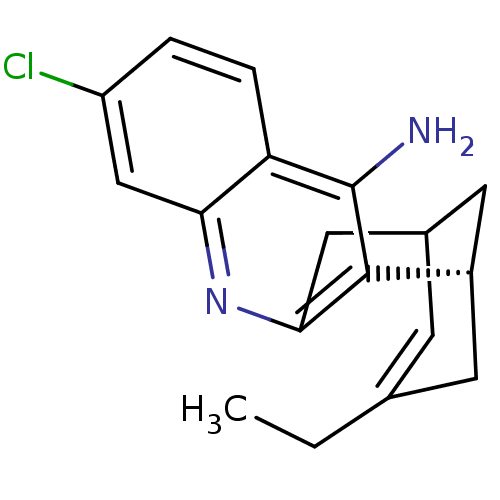
(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271471
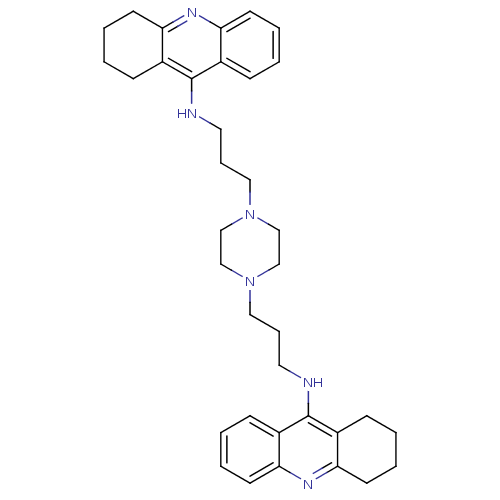
(1,4-bis[3-(1,2,3,4-Tetrahydroacridin-9-yl)aminopro...)Show SMILES C(CNc1c2CCCCc2nc2ccccc12)CN1CCN(CCCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C36H46N6/c1-5-15-31-27(11-1)35(28-12-2-6-16-32(28)39-31)37-19-9-21-41-23-25-42(26-24-41)22-10-20-38-36-29-13-3-7-17-33(29)40-34-18-8-4-14-30(34)36/h1,3,5,7,11,13,15,17H,2,4,6,8-10,12,14,16,18-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271468
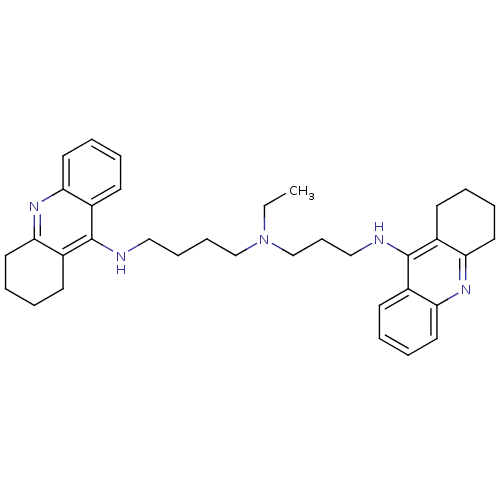
(CHEMBL490060 | N-Ethyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5/c1-2-40(25-13-23-37-35-28-16-5-9-20-32(28)39-33-21-10-6-17-29(33)35)24-12-11-22-36-34-26-14-3-7-18-30(26)38-31-19-8-4-15-27(31)34/h3,5,7,9,14,16,18,20H,2,4,6,8,10-13,15,17,19,21-25H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271325
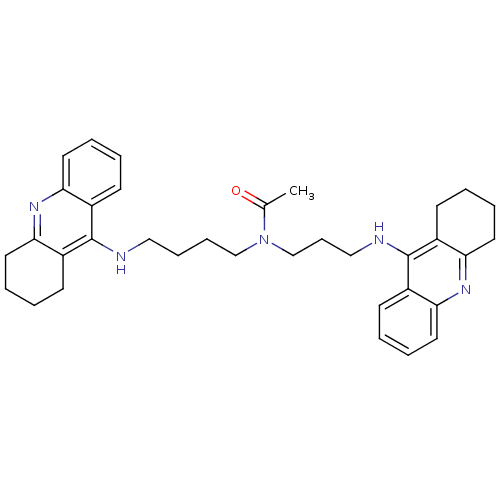
(CHEMBL451277 | N-{4-[(1,2,3,4-Tetrahydroacridin-9-...)Show SMILES CC(=O)N(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N5O/c1-25(41)40(24-12-22-37-35-28-15-4-8-19-32(28)39-33-20-9-5-16-29(33)35)23-11-10-21-36-34-26-13-2-6-17-30(26)38-31-18-7-3-14-27(31)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271323
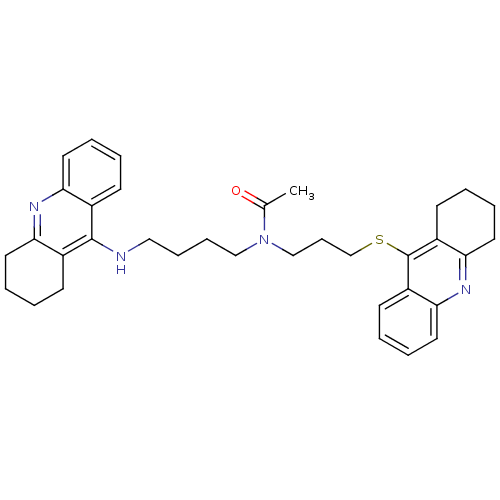
(CHEMBL501587 | N-{4-[(1,2,3, 4-Tetrahydroacridin-9...)Show SMILES CC(=O)N(CCCCNc1c2CCCCc2nc2ccccc12)CCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H42N4OS/c1-25(40)39(23-12-24-41-35-28-15-4-8-19-32(28)38-33-20-9-5-16-29(33)35)22-11-10-21-36-34-26-13-2-6-17-30(26)37-31-18-7-3-14-27(31)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50149201

(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to mouse AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271468

(CHEMBL490060 | N-Ethyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5/c1-2-40(25-13-23-37-35-28-16-5-9-20-32(28)39-33-21-10-6-17-29(33)35)24-12-11-22-36-34-26-14-3-7-18-30(26)38-31-19-8-4-15-27(31)34/h3,5,7,9,14,16,18,20H,2,4,6,8,10-13,15,17,19,21-25H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50377920
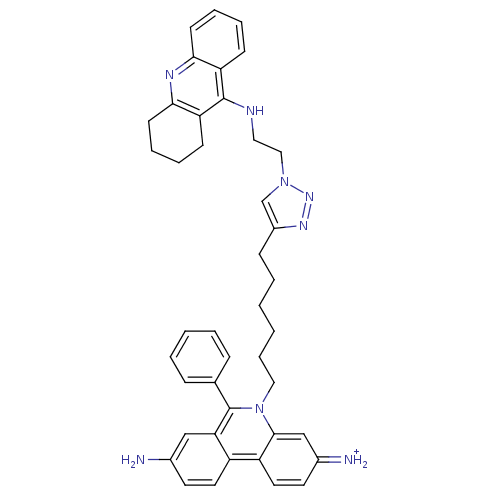
(CHEMBL540657)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cn(CCNc3c4CCCCc4nc4ccccc34)nn1)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)50(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-49(48-47-32)25-23-45-41-35-15-7-9-17-38(35)46-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,46)/p+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of mouse BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8965
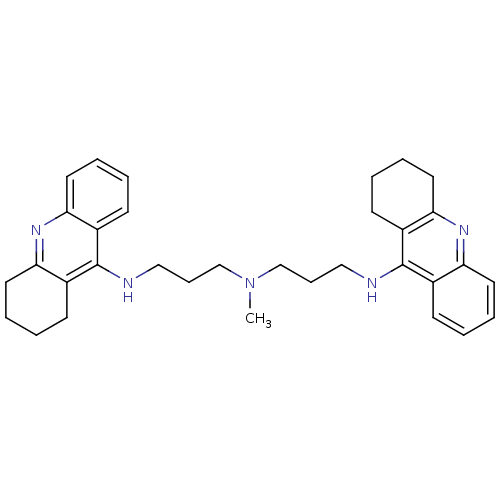
(CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...)Show SMILES CN(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H41N5/c1-38(22-10-20-34-32-24-12-2-6-16-28(24)36-29-17-7-3-13-25(29)32)23-11-21-35-33-26-14-4-8-18-30(26)37-31-19-9-5-15-27(31)33/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,34,36)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50369748

(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50377920

(CHEMBL540657)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cn(CCNc3c4CCCCc4nc4ccccc34)nn1)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)50(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-49(48-47-32)25-23-45-41-35-15-7-9-17-38(35)46-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,46)/p+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50149201

(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of mouse BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271325

(CHEMBL451277 | N-{4-[(1,2,3,4-Tetrahydroacridin-9-...)Show SMILES CC(=O)N(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N5O/c1-25(41)40(24-12-22-37-35-28-15-4-8-19-32(28)39-33-20-9-5-16-29(33)35)23-11-10-21-36-34-26-13-2-6-17-30(26)38-31-18-7-3-14-27(31)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.725 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8977
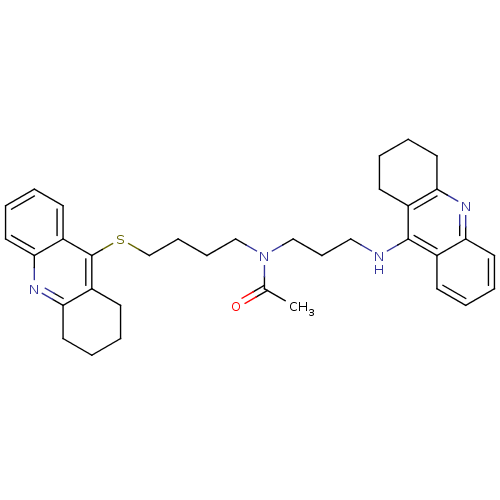
(CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...)Show SMILES CC(=O)N(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H42N4OS/c1-25(40)39(23-12-21-36-34-26-13-2-6-17-30(26)37-31-18-7-3-14-27(31)34)22-10-11-24-41-35-28-15-4-8-19-32(28)38-33-20-9-5-16-29(33)35/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271473
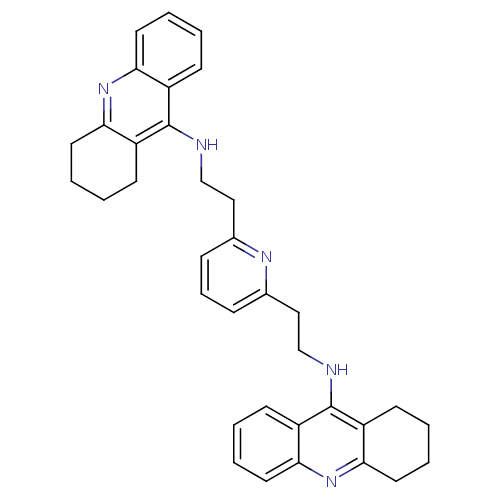
(2,6-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1cccc(CCNc2c3CCCCc3nc3ccccc23)n1)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H37N5/c1-5-16-30-26(12-1)34(27-13-2-6-17-31(27)39-30)36-22-20-24-10-9-11-25(38-24)21-23-37-35-28-14-3-7-18-32(28)40-33-19-8-4-15-29(33)35/h1,3,5,7,9-12,14,16,18H,2,4,6,8,13,15,17,19-23H2,(H,36,39)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.768 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271324
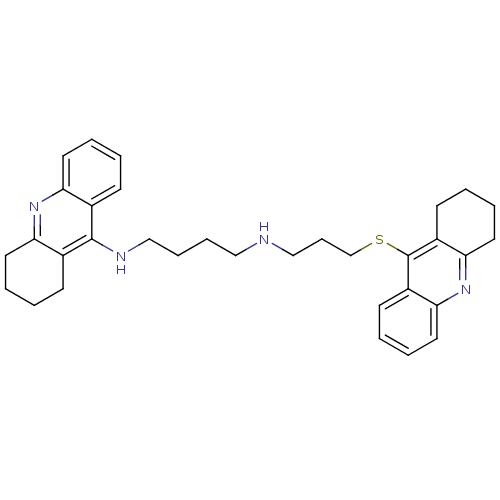
(CHEMBL482512 | N-(1,2,3,4-Tetrahydroacridin-9-yl)-...)Show SMILES C(CCNc1c2CCCCc2nc2ccccc12)CNCCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4S/c1-5-16-28-24(12-1)32(25-13-2-6-17-29(25)36-28)35-22-10-9-20-34-21-11-23-38-33-26-14-3-7-18-30(26)37-31-19-8-4-15-27(31)33/h1,3,5,7,12,14,16,18,34H,2,4,6,8-11,13,15,17,19-23H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.779 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10439
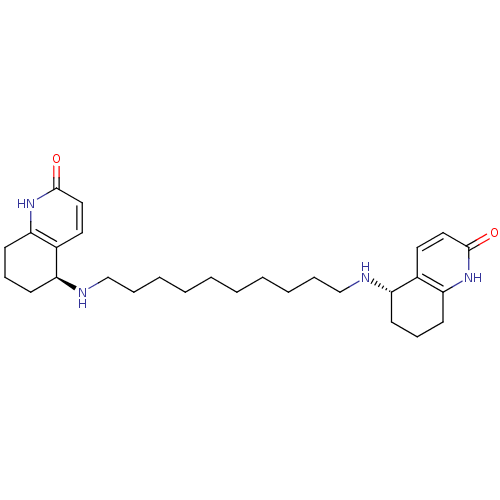
((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...)Show SMILES O=c1ccc2[C@H](CCCc2[nH]1)NCCCCCCCCCCN[C@H]1CCCc2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C28H42N4O2/c33-27-17-15-21-23(11-9-13-25(21)31-27)29-19-7-5-3-1-2-4-6-8-20-30-24-12-10-14-26-22(24)16-18-28(34)32-26/h15-18,23-24,29-30H,1-14,19-20H2,(H,31,33)(H,32,34)/t23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271367

(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.819 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271472
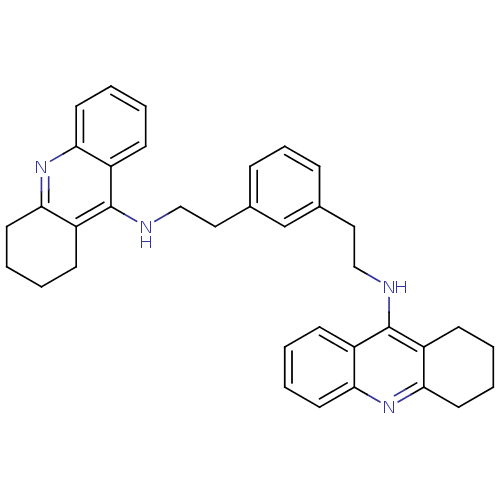
(1,3-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1cccc(CCNc2c3CCCCc3nc3ccccc23)c1)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H38N4/c1-5-16-31-27(12-1)35(28-13-2-6-17-32(28)39-31)37-22-20-25-10-9-11-26(24-25)21-23-38-36-29-14-3-7-18-33(29)40-34-19-8-4-15-30(34)36/h1,3,5,7,9-12,14,16,18,24H,2,4,6,8,13,15,17,19-23H2,(H,37,39)(H,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.924 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271470

(CHEMBL499224 | N-(2-Hydroxyethyl)-N-(1,2,3,4-tetra...)Show SMILES OCCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5O/c41-25-24-40(23-11-21-37-35-28-14-3-7-18-32(28)39-33-19-8-4-15-29(33)35)22-10-9-20-36-34-26-12-1-5-16-30(26)38-31-17-6-2-13-27(31)34/h1,3,5,7,12,14,16,18,41H,2,4,6,8-11,13,15,17,19-25H2,(H,36,38)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of fetal bovine serum AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271517
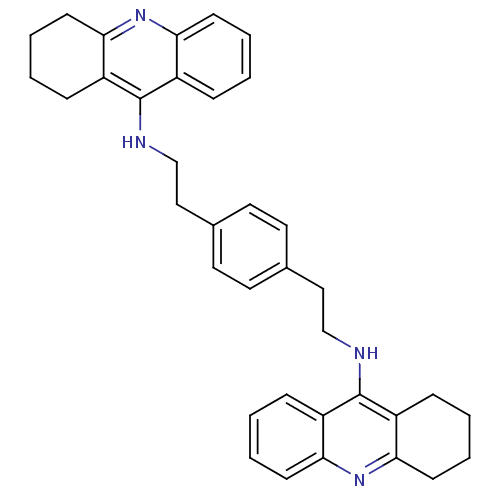
(1,4-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1ccc(CCNc2c3CCCCc3nc3ccccc23)cc1)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H38N4/c1-5-13-31-27(9-1)35(28-10-2-6-14-32(28)39-31)37-23-21-25-17-19-26(20-18-25)22-24-38-36-29-11-3-7-15-33(29)40-34-16-8-4-12-30(34)36/h1,3,5,7,9,11,13,15,17-20H,2,4,6,8,10,12,14,16,21-24H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8971
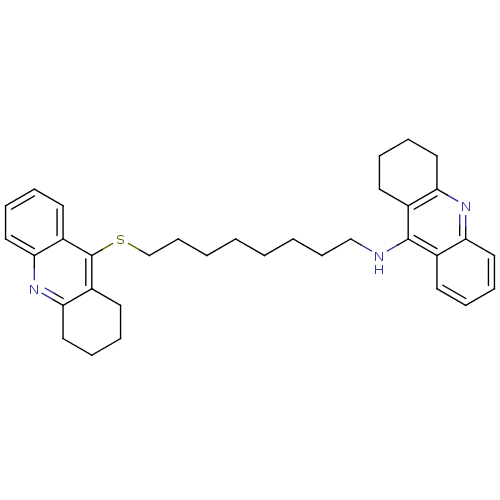
(CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...)Show SMILES C(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3S/c1(3-13-23-35-33-25-15-5-9-19-29(25)36-30-20-10-6-16-26(30)33)2-4-14-24-38-34-27-17-7-11-21-31(27)37-32-22-12-8-18-28(32)34/h5,7,9,11,15,17,19,21H,1-4,6,8,10,12-14,16,18,20,22-24H2,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271472

(1,3-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1cccc(CCNc2c3CCCCc3nc3ccccc23)c1)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H38N4/c1-5-16-31-27(12-1)35(28-13-2-6-17-32(28)39-31)37-22-20-25-10-9-11-26(24-25)21-23-38-36-29-14-3-7-18-33(29)40-34-19-8-4-15-30(34)36/h1,3,5,7,9-12,14,16,18,24H,2,4,6,8,13,15,17,19-23H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271473

(2,6-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1cccc(CCNc2c3CCCCc3nc3ccccc23)n1)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H37N5/c1-5-16-30-26(12-1)34(27-13-2-6-17-31(27)39-30)36-22-20-24-10-9-11-25(38-24)21-23-37-35-28-14-3-7-18-32(28)40-33-19-8-4-15-29(33)35/h1,3,5,7,9-12,14,16,18H,2,4,6,8,13,15,17,19-23H2,(H,36,39)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of horse BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271324

(CHEMBL482512 | N-(1,2,3,4-Tetrahydroacridin-9-yl)-...)Show SMILES C(CCNc1c2CCCCc2nc2ccccc12)CNCCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4S/c1-5-16-28-24(12-1)32(25-13-2-6-17-29(25)36-28)35-22-10-9-20-34-21-11-23-38-33-26-14-3-7-18-30(26)37-31-19-8-4-15-27(31)33/h1,3,5,7,12,14,16,18,34H,2,4,6,8-11,13,15,17,19-23H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8975
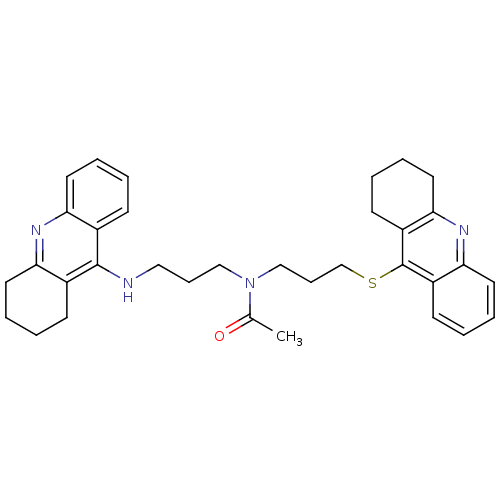
(CHEMBL179192 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...)Show SMILES CC(=O)N(CCCNc1c2CCCCc2nc2ccccc12)CCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H40N4OS/c1-24(39)38(21-10-20-35-33-25-12-2-6-16-29(25)36-30-17-7-3-13-26(30)33)22-11-23-40-34-27-14-4-8-18-31(27)37-32-19-9-5-15-28(32)34/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271471

(1,4-bis[3-(1,2,3,4-Tetrahydroacridin-9-yl)aminopro...)Show SMILES C(CNc1c2CCCCc2nc2ccccc12)CN1CCN(CCCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C36H46N6/c1-5-15-31-27(11-1)35(28-12-2-6-16-32(28)39-31)37-19-9-21-41-23-25-42(26-24-41)22-10-20-38-36-29-13-3-7-17-33(29)40-34-18-8-4-14-30(34)36/h1,3,5,7,11,13,15,17H,2,4,6,8-10,12,14,16,18-26H2,(H,37,39)(H,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8976
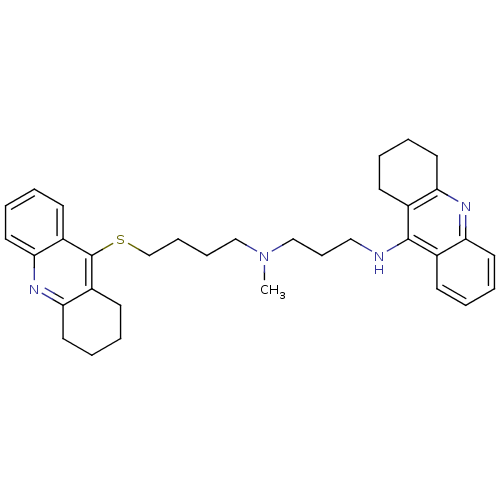
(CHEMBL175555 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H42N4S/c1-38(23-12-21-35-33-25-13-2-6-17-29(25)36-30-18-7-3-14-26(30)33)22-10-11-24-39-34-27-15-4-8-19-31(27)37-32-20-9-5-16-28(32)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271469

(CHEMBL507174 | N-Allyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES C=CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H45N5/c1-2-24-41(26-13-23-38-36-29-16-5-9-20-33(29)40-34-21-10-6-17-30(34)36)25-12-11-22-37-35-27-14-3-7-18-31(27)39-32-19-8-4-15-28(32)35/h2-3,5,7,9,14,16,18,20H,1,4,6,8,10-13,15,17,19,21-26H2,(H,37,39)(H,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8974
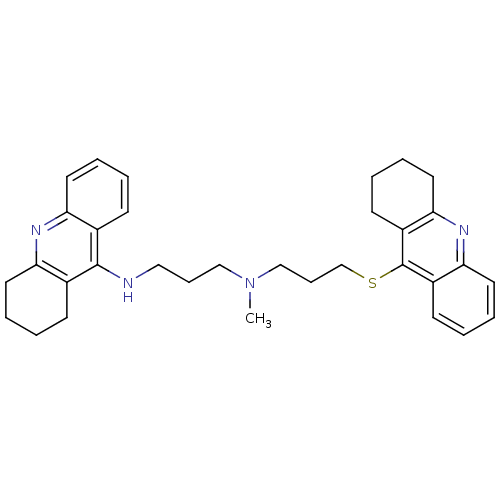
(CHEMBL367067 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCNc1c2CCCCc2nc2ccccc12)CCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4S/c1-37(21-10-20-34-32-24-12-2-6-16-28(24)35-29-17-7-3-13-25(29)32)22-11-23-38-33-26-14-4-8-18-30(26)36-31-19-9-5-15-27(31)33/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of horse BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440
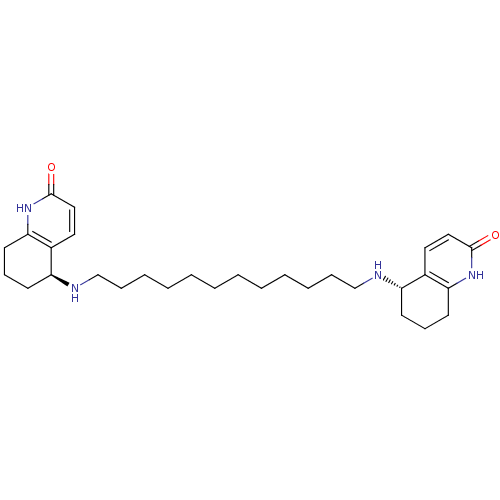
((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...)Show SMILES O=c1ccc2[C@H](CCCc2[nH]1)NCCCCCCCCCCCCN[C@H]1CCCc2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C30H46N4O2/c35-29-19-17-23-25(13-11-15-27(23)33-29)31-21-9-7-5-3-1-2-4-6-8-10-22-32-26-14-12-16-28-24(26)18-20-30(36)34-28/h17-20,25-26,31-32H,1-16,21-22H2,(H,33,35)(H,34,36)/t25-,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440

((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...)Show SMILES O=c1ccc2[C@H](CCCc2[nH]1)NCCCCCCCCCCCCN[C@H]1CCCc2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C30H46N4O2/c35-29-19-17-23-25(13-11-15-27(23)33-29)31-21-9-7-5-3-1-2-4-6-8-10-22-32-26-14-12-16-28-24(26)18-20-30(36)34-28/h17-20,25-26,31-32H,1-16,21-22H2,(H,33,35)(H,34,36)/t25-,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271518
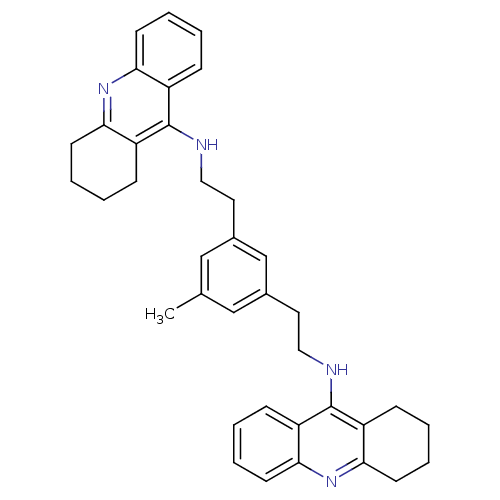
(1,3-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES Cc1cc(CCNc2c3CCCCc3nc3ccccc23)cc(CCNc2c3CCCCc3nc3ccccc23)c1 Show InChI InChI=1S/C37H40N4/c1-25-22-26(18-20-38-36-28-10-2-6-14-32(28)40-33-15-7-3-11-29(33)36)24-27(23-25)19-21-39-37-30-12-4-8-16-34(30)41-35-17-9-5-13-31(35)37/h2,4,6,8,10,12,14,16,22-24H,3,5,7,9,11,13,15,17-21H2,1H3,(H,38,40)(H,39,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8977

(CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...)Show SMILES CC(=O)N(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H42N4OS/c1-25(40)39(23-12-21-36-34-26-13-2-6-17-30(26)37-31-18-7-3-14-27(31)34)22-10-11-24-41-35-28-15-4-8-19-32(28)38-33-20-9-5-16-29(33)35/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50271519
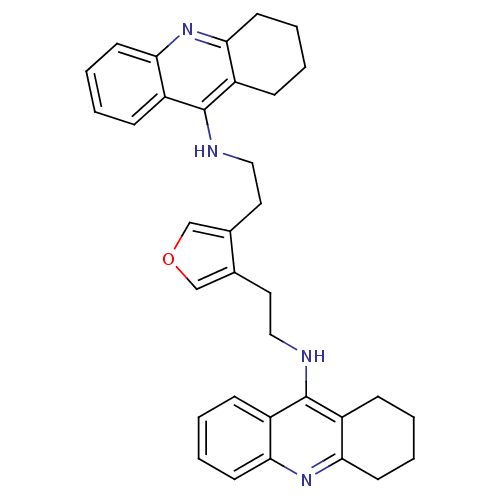
(3,4-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1cocc1CCNc1c2CCCCc2nc2ccccc12)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H36N4O/c1-5-13-29-25(9-1)33(26-10-2-6-14-30(26)37-29)35-19-17-23-21-39-22-24(23)18-20-36-34-27-11-3-7-15-31(27)38-32-16-8-4-12-28(32)34/h1,3,5,7,9,11,13,15,21-22H,2,4,6,8,10,12,14,16-20H2,(H,35,37)(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271518

(1,3-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES Cc1cc(CCNc2c3CCCCc3nc3ccccc23)cc(CCNc2c3CCCCc3nc3ccccc23)c1 Show InChI InChI=1S/C37H40N4/c1-25-22-26(18-20-38-36-28-10-2-6-14-32(28)40-33-15-7-3-11-29(33)36)24-27(23-25)19-21-39-37-30-12-4-8-16-34(30)41-35-17-9-5-13-31(35)37/h2,4,6,8,10,12,14,16,22-24H,3,5,7,9,11,13,15,17-21H2,1H3,(H,38,40)(H,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8976

(CHEMBL175555 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCSc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H42N4S/c1-38(23-12-21-35-33-25-13-2-6-17-29(25)36-30-18-7-3-14-26(30)33)22-10-11-24-39-34-27-15-4-8-19-31(27)37-32-20-9-5-16-28(32)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50377920

(CHEMBL540657)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cn(CCNc3c4CCCCc4nc4ccccc34)nn1)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)50(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-49(48-47-32)25-23-45-41-35-15-7-9-17-38(35)46-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,46)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to mouse AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8974

(CHEMBL367067 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCNc1c2CCCCc2nc2ccccc12)CCCSc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4S/c1-37(21-10-20-34-32-24-12-2-6-16-28(24)35-29-17-7-3-13-25(29)32)22-11-23-38-33-26-14-4-8-18-30(26)36-31-19-9-5-15-27(31)33/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of fetal bovine serum AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8965

(CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...)Show SMILES CN(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H41N5/c1-38(22-10-20-34-32-24-12-2-6-16-28(24)36-29-17-7-3-13-25(29)32)23-11-21-35-33-26-14-4-8-18-30(26)37-31-19-9-5-15-27(31)33/h2,4,6,8,12,14,16,18H,3,5,7,9-11,13,15,17,19-23H2,1H3,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271519

(3,4-bis{[(1,2,3,4-Tetrahydroacridin-9-yl)amino]eth...)Show SMILES C(Cc1cocc1CCNc1c2CCCCc2nc2ccccc12)Nc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H36N4O/c1-5-13-29-25(9-1)33(26-10-2-6-14-30(26)37-29)35-19-17-23-21-39-22-24(23)18-20-36-34-27-11-3-7-15-31(27)38-32-16-8-4-12-28(32)34/h1,3,5,7,9,11,13,15,21-22H,2,4,6,8,10,12,14,16-20H2,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data