 Found 45 hits Enz. Inhib. hit(s) with all data for entry = 50038645
Found 45 hits Enz. Inhib. hit(s) with all data for entry = 50038645 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262340

(11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...)Show SMILES Cc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:18| Show InChI InChI=1S/C25H29N3O/c1-14-8-10-15(11-9-14)20-21-18(12-25(2,3)13-19(21)29)28-24-22(20)23(26)16-6-4-5-7-17(16)27-24/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262341
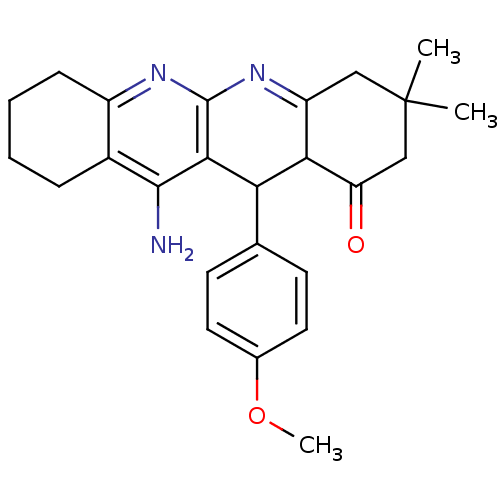
(11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...)Show SMILES COc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:19| Show InChI InChI=1S/C25H29N3O2/c1-25(2)12-18-21(19(29)13-25)20(14-8-10-15(30-3)11-9-14)22-23(26)16-6-4-5-7-17(16)27-24(22)28-18/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262282
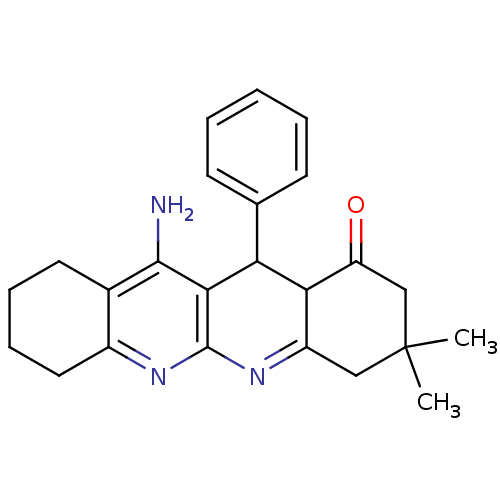
(11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...)Show SMILES CC1(C)CC(=O)C2C(c3ccccc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C24H27N3O/c1-24(2)12-17-20(18(28)13-24)19(14-8-4-3-5-9-14)21-22(25)15-10-6-7-11-16(15)26-23(21)27-17/h3-5,8-9,19-20H,6-7,10-13H2,1-2H3,(H2,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262339
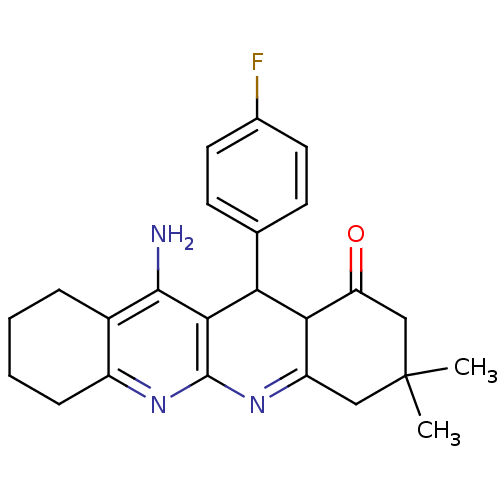
(11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...)Show SMILES CC1(C)CC(=O)C2C(c3ccc(F)cc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:29| Show InChI InChI=1S/C24H26FN3O/c1-24(2)11-17-20(18(29)12-24)19(13-7-9-14(25)10-8-13)21-22(26)15-5-3-4-6-16(15)27-23(21)28-17/h7-10,19-20H,3-6,11-12H2,1-2H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262342
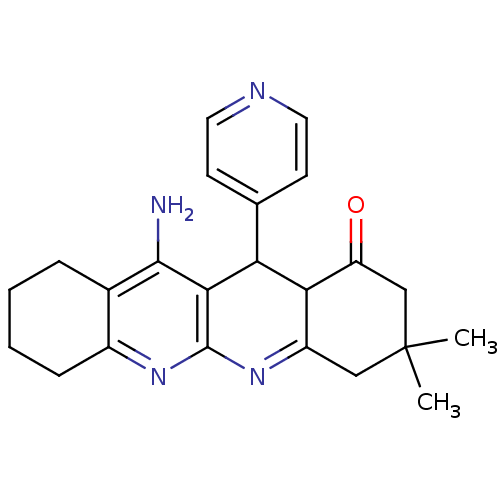
(11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...)Show SMILES CC1(C)CC(=O)C2C(c3ccncc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C23H26N4O/c1-23(2)11-16-19(17(28)12-23)18(13-7-9-25-10-8-13)20-21(24)14-5-3-4-6-15(14)26-22(20)27-16/h7-10,18-19H,3-6,11-12H2,1-2H3,(H2,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM31904
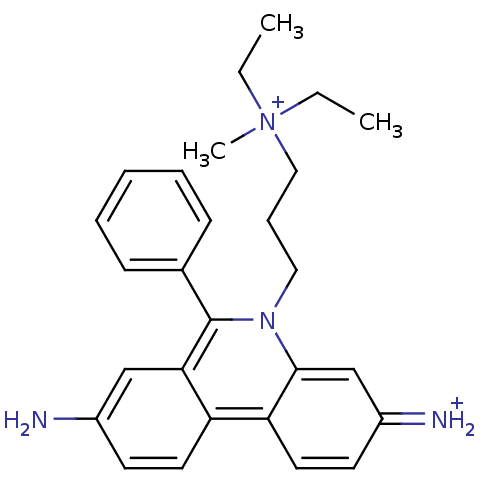
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM29400
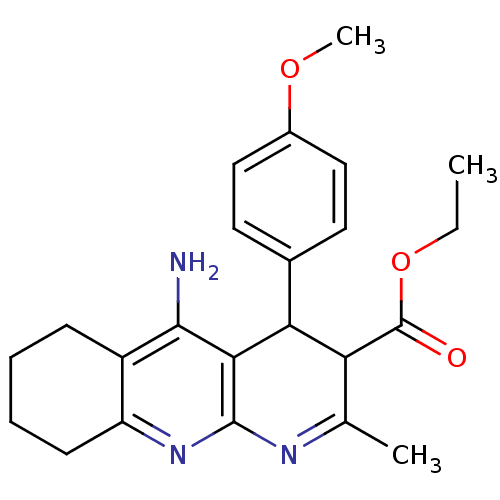
(CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(OC)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:29| Show InChI InChI=1S/C23H27N3O3/c1-4-29-23(27)18-13(2)25-22-20(19(18)14-9-11-15(28-3)12-10-14)21(24)16-7-5-6-8-17(16)26-22/h9-12,18-19H,4-8H2,1-3H3,(H2,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM29391
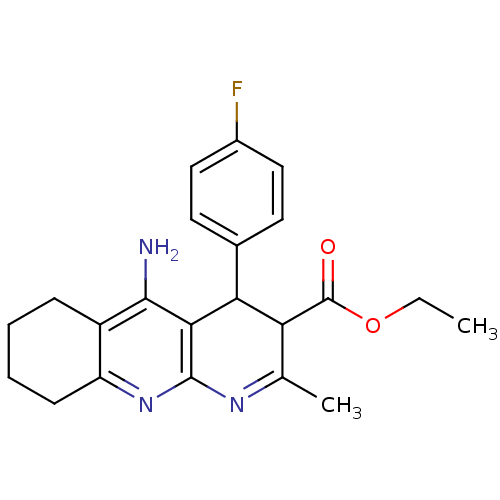
(CHEMBL219172 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(F)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:28| Show InChI InChI=1S/C22H24FN3O2/c1-3-28-22(27)17-12(2)25-21-19(18(17)13-8-10-14(23)11-9-13)20(24)15-6-4-5-7-16(15)26-21/h8-11,17-18H,3-7H2,1-2H3,(H2,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM29390
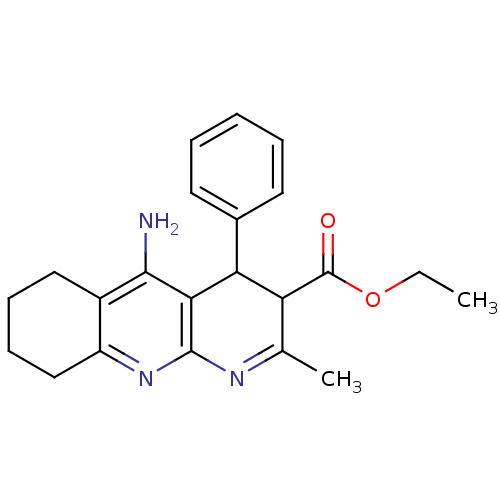
(CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccccc2)c2c(N)c3CCCCc3nc2N=C1C |c:27| Show InChI InChI=1S/C22H25N3O2/c1-3-27-22(26)17-13(2)24-21-19(18(17)14-9-5-4-6-10-14)20(23)15-11-7-8-12-16(15)25-21/h4-6,9-10,17-18H,3,7-8,11-12H2,1-2H3,(H2,23,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM29396
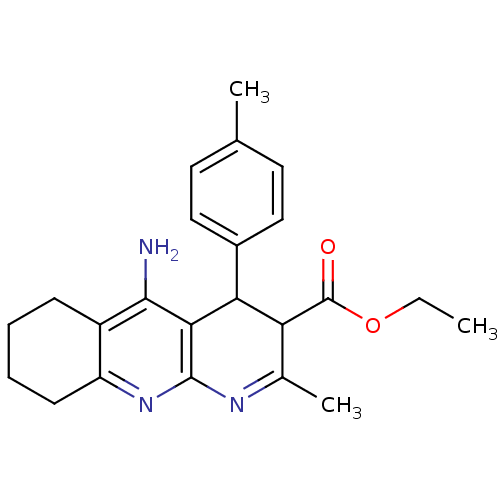
(CHEMBL219406 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(C)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:28| Show InChI InChI=1S/C23H27N3O2/c1-4-28-23(27)18-14(3)25-22-20(19(18)15-11-9-13(2)10-12-15)21(24)16-7-5-6-8-17(16)26-22/h9-12,18-19H,4-8H2,1-3H3,(H2,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM29400

(CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(OC)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:29| Show InChI InChI=1S/C23H27N3O3/c1-4-29-23(27)18-13(2)25-22-20(19(18)14-9-11-15(28-3)12-10-14)21(24)16-7-5-6-8-17(16)26-22/h9-12,18-19H,4-8H2,1-3H3,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM29390

(CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccccc2)c2c(N)c3CCCCc3nc2N=C1C |c:27| Show InChI InChI=1S/C22H25N3O2/c1-3-27-22(26)17-13(2)24-21-19(18(17)14-9-5-4-6-10-14)20(23)15-11-7-8-12-16(15)25-21/h4-6,9-10,17-18H,3,7-8,11-12H2,1-2H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM29396

(CHEMBL219406 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(C)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:28| Show InChI InChI=1S/C23H27N3O2/c1-4-28-23(27)18-14(3)25-22-20(19(18)15-11-9-13(2)10-12-15)21(24)16-7-5-6-8-17(16)26-22/h9-12,18-19H,4-8H2,1-3H3,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM29391

(CHEMBL219172 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(F)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:28| Show InChI InChI=1S/C22H24FN3O2/c1-3-28-22(27)17-12(2)25-21-19(18(17)13-8-10-14(23)11-9-13)20(24)15-6-4-5-7-16(15)26-21/h8-11,17-18H,3-7H2,1-2H3,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262341

(11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...)Show SMILES COc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:19| Show InChI InChI=1S/C25H29N3O2/c1-25(2)12-18-21(19(29)13-25)20(14-8-10-15(30-3)11-9-14)22-23(26)16-6-4-5-7-17(16)27-24(22)28-18/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262340

(11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...)Show SMILES Cc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:18| Show InChI InChI=1S/C25H29N3O/c1-14-8-10-15(11-9-14)20-21-18(12-25(2,3)13-19(21)29)28-24-22(20)23(26)16-6-4-5-7-17(16)27-24/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262282

(11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...)Show SMILES CC1(C)CC(=O)C2C(c3ccccc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C24H27N3O/c1-24(2)12-17-20(18(28)13-24)19(14-8-4-3-5-9-14)21-22(25)15-10-6-7-11-16(15)26-23(21)27-17/h3-5,8-9,19-20H,6-7,10-13H2,1-2H3,(H2,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262341

(11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...)Show SMILES COc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:19| Show InChI InChI=1S/C25H29N3O2/c1-25(2)12-18-21(19(29)13-25)20(14-8-10-15(30-3)11-9-14)22-23(26)16-6-4-5-7-17(16)27-24(22)28-18/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262339

(11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...)Show SMILES CC1(C)CC(=O)C2C(c3ccc(F)cc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:29| Show InChI InChI=1S/C24H26FN3O/c1-24(2)11-17-20(18(29)12-24)19(13-7-9-14(25)10-8-13)21-22(26)15-5-3-4-6-16(15)27-23(21)28-17/h7-10,19-20H,3-6,11-12H2,1-2H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262342

(11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...)Show SMILES CC1(C)CC(=O)C2C(c3ccncc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C23H26N4O/c1-23(2)11-16-19(17(28)12-23)18(13-7-9-25-10-8-13)20-21(24)14-5-3-4-6-15(14)26-22(20)27-16/h7-10,18-19H,3-6,11-12H2,1-2H3,(H2,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262340

(11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...)Show SMILES Cc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:18| Show InChI InChI=1S/C25H29N3O/c1-14-8-10-15(11-9-14)20-21-18(12-25(2,3)13-19(21)29)28-24-22(20)23(26)16-6-4-5-7-17(16)27-24/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262282

(11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...)Show SMILES CC1(C)CC(=O)C2C(c3ccccc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C24H27N3O/c1-24(2)12-17-20(18(28)13-24)19(14-8-4-3-5-9-14)21-22(25)15-10-6-7-11-16(15)26-23(21)27-17/h3-5,8-9,19-20H,6-7,10-13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50241350
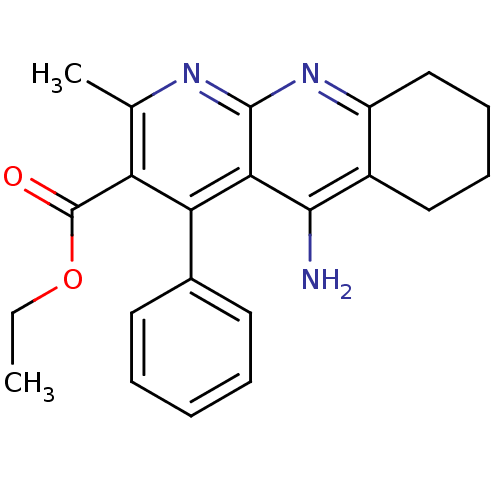
(5-Amino-2-methyl-4-phenyl-6,7,8,9-tetrahydro-benzo...)Show SMILES CCOC(=O)c1c(C)nc2nc3CCCCc3c(N)c2c1-c1ccccc1 Show InChI InChI=1S/C22H23N3O2/c1-3-27-22(26)17-13(2)24-21-19(18(17)14-9-5-4-6-10-14)20(23)15-11-7-8-12-16(15)25-21/h4-6,9-10H,3,7-8,11-12H2,1-2H3,(H2,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50241346
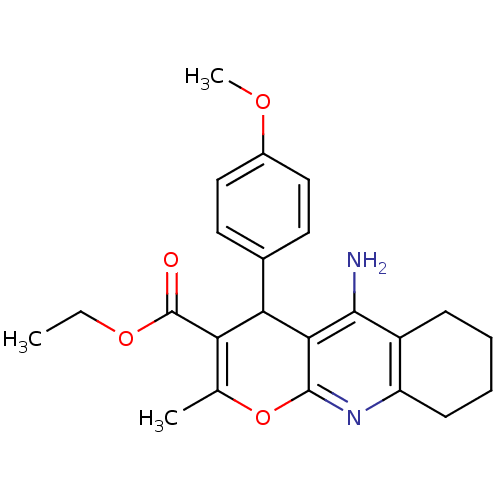
(10-Amino-4-(4-methoxy-phenyl)-2-methyl-5,6,7,8-tet...)Show SMILES CCOC(=O)C1=C(C)Oc2nc3CCCCc3c(N)c2C1c1ccc(OC)cc1 |c:5| Show InChI InChI=1S/C23H26N2O4/c1-4-28-23(26)18-13(2)29-22-20(19(18)14-9-11-15(27-3)12-10-14)21(24)16-7-5-6-8-17(16)25-22/h9-12,19H,4-8H2,1-3H3,(H2,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50241351

(5-Amino-2-methyl-4-pyridin-4-yl-6,7,8,9-tetrahydro...)Show SMILES CCOC(=O)c1c(C)nc2nc3CCCCc3c(N)c2c1-c1ccncc1 Show InChI InChI=1S/C21H22N4O2/c1-3-27-21(26)16-12(2)24-20-18(17(16)13-8-10-23-11-9-13)19(22)14-6-4-5-7-15(14)25-20/h8-11H,3-7H2,1-2H3,(H2,22,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262339

(11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...)Show SMILES CC1(C)CC(=O)C2C(c3ccc(F)cc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:29| Show InChI InChI=1S/C24H26FN3O/c1-24(2)11-17-20(18(29)12-24)19(13-7-9-14(25)10-8-13)21-22(26)15-5-3-4-6-16(15)27-23(21)28-17/h7-10,19-20H,3-6,11-12H2,1-2H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50262342

(11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...)Show SMILES CC1(C)CC(=O)C2C(c3ccncc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C23H26N4O/c1-23(2)11-16-19(17(28)12-23)18(13-7-9-25-10-8-13)20-21(24)14-5-3-4-6-15(14)26-22(20)27-16/h7-10,18-19H,3-6,11-12H2,1-2H3,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50241349
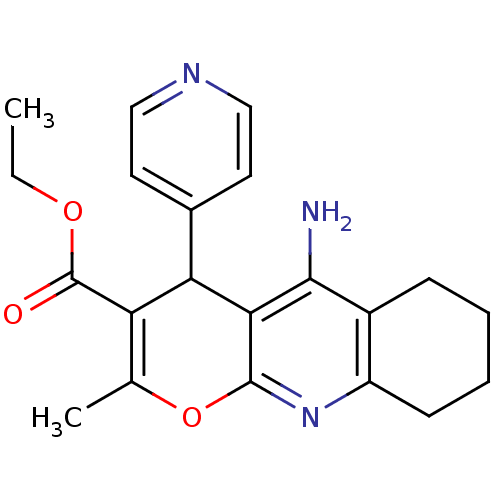
(10-Amino-2-methyl-4-pyridin-4-yl-5,6,7,8-tetrahydr...)Show SMILES CCOC(=O)C1=C(C)Oc2nc3CCCCc3c(N)c2C1c1ccncc1 |c:5| Show InChI InChI=1S/C21H23N3O3/c1-3-26-21(25)16-12(2)27-20-18(17(16)13-8-10-23-11-9-13)19(22)14-6-4-5-7-15(14)24-20/h8-11,17H,3-7H2,1-2H3,(H2,22,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262511
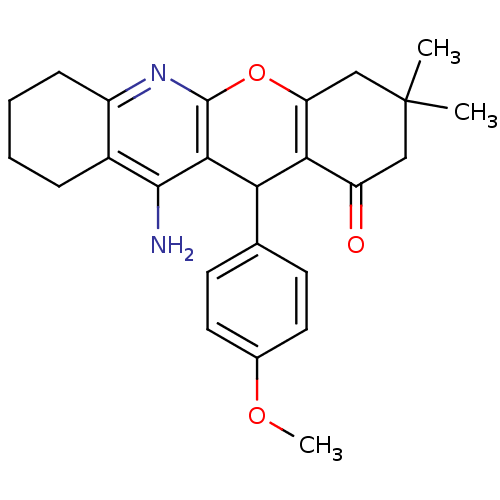
(11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-2,3,4,...)Show SMILES COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)Oc2nc3CCCCc3c(N)c12 |t:10| Show InChI InChI=1S/C25H28N2O3/c1-25(2)12-18(28)21-19(13-25)30-24-22(20(21)14-8-10-15(29-3)11-9-14)23(26)16-6-4-5-7-17(16)27-24/h8-11,20H,4-7,12-13H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262454

(11-Amino-3,3-dimethyl-12-phenyl-2,3,4,7,8,9,10,12-...)Show SMILES CC1(C)CC(=O)C2=C(C1)Oc1nc3CCCCc3c(N)c1C2c1ccccc1 |c:6| Show InChI InChI=1S/C24H26N2O2/c1-24(2)12-17(27)20-18(13-24)28-23-21(19(20)14-8-4-3-5-9-14)22(25)15-10-6-7-11-16(15)26-23/h3-5,8-9,19H,6-7,10-13H2,1-2H3,(H2,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262456
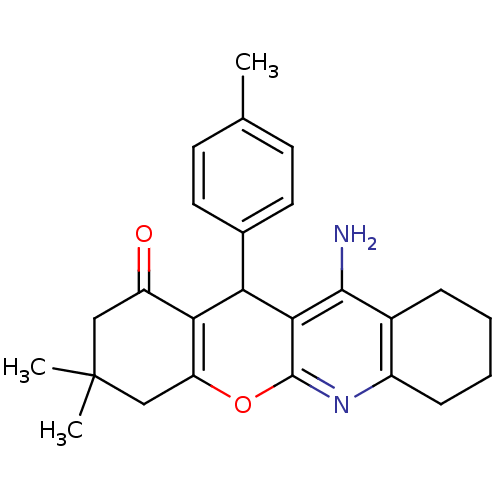
(11-Amino-3,3-dimethyl-12-p-tolyl-2,3,4,7,8,9,10,12...)Show SMILES Cc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)Oc2nc3CCCCc3c(N)c12 |t:9| Show InChI InChI=1S/C25H28N2O2/c1-14-8-10-15(11-9-14)20-21-18(28)12-25(2,3)13-19(21)29-24-22(20)23(26)16-6-4-5-7-17(16)27-24/h8-11,20H,4-7,12-13H2,1-3H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50262455
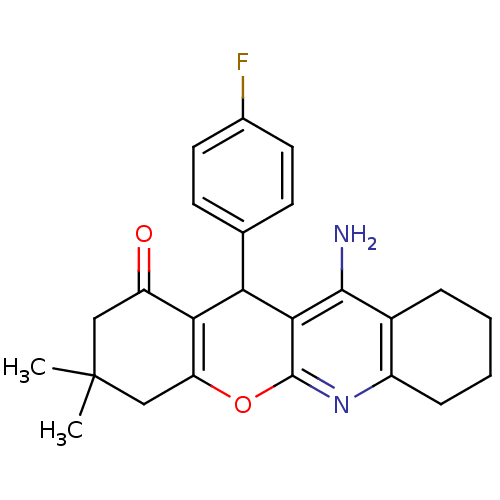
(11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-2,3,4,7...)Show SMILES CC1(C)CC(=O)C2=C(C1)Oc1nc3CCCCc3c(N)c1C2c1ccc(F)cc1 |c:6| Show InChI InChI=1S/C24H25FN2O2/c1-24(2)11-17(28)20-18(12-24)29-23-21(19(20)13-7-9-14(25)10-8-13)22(26)15-5-3-4-6-16(15)27-23/h7-10,19H,3-6,11-12H2,1-2H3,(H2,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE by spectrophotometry |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50262282

(11-Amino-3,3-dimethyl-12-phenyl-3,4,5,7,8,9,10,12-...)Show SMILES CC1(C)CC(=O)C2C(c3ccccc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C24H27N3O/c1-24(2)12-17-20(18(28)13-24)19(14-8-4-3-5-9-14)21-22(25)15-10-6-7-11-16(15)26-23(21)27-17/h3-5,8-9,19-20H,6-7,10-13H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM29400

(CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(OC)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:29| Show InChI InChI=1S/C23H27N3O3/c1-4-29-23(27)18-13(2)25-22-20(19(18)14-9-11-15(28-3)12-10-14)21(24)16-7-5-6-8-17(16)26-22/h9-12,18-19H,4-8H2,1-3H3,(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM29396

(CHEMBL219406 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(C)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:28| Show InChI InChI=1S/C23H27N3O2/c1-4-28-23(27)18-14(3)25-22-20(19(18)15-11-9-13(2)10-12-15)21(24)16-7-5-6-8-17(16)26-22/h9-12,18-19H,4-8H2,1-3H3,(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM29391

(CHEMBL219172 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccc(F)cc2)c2c(N)c3CCCCc3nc2N=C1C |c:28| Show InChI InChI=1S/C22H24FN3O2/c1-3-28-22(27)17-12(2)25-21-19(18(17)13-8-10-14(23)11-9-13)20(24)15-6-4-5-7-16(15)26-21/h8-11,17-18H,3-7H2,1-2H3,(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM29390

(CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...)Show SMILES CCOC(=O)C1C(c2ccccc2)c2c(N)c3CCCCc3nc2N=C1C |c:27| Show InChI InChI=1S/C22H25N3O2/c1-3-27-22(26)17-13(2)24-21-19(18(17)14-9-5-4-6-10-14)20(23)15-11-7-8-12-16(15)25-21/h4-6,9-10,17-18H,3,7-8,11-12H2,1-2H3,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50262342

(11-Amino-3,3-dimethyl-12-pyridin-4-yl-3,4,5,7,8,9,...)Show SMILES CC1(C)CC(=O)C2C(c3ccncc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:28| Show InChI InChI=1S/C23H26N4O/c1-23(2)11-16-19(17(28)12-23)18(13-7-9-25-10-8-13)20-21(24)14-5-3-4-6-15(14)26-22(20)27-16/h7-10,18-19H,3-6,11-12H2,1-2H3,(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50262340

(11-Amino-3,3-dimethyl-12-p-tolyl-3,4,5,7,8,9,10,12...)Show SMILES Cc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:18| Show InChI InChI=1S/C25H29N3O/c1-14-8-10-15(11-9-14)20-21-18(12-25(2,3)13-19(21)29)28-24-22(20)23(26)16-6-4-5-7-17(16)27-24/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50262339

(11-Amino-12-(4-fluoro-phenyl)-3,3-dimethyl-3,4,5,7...)Show SMILES CC1(C)CC(=O)C2C(c3ccc(F)cc3)c3c(N)c4CCCCc4nc3N=C2C1 |c:29| Show InChI InChI=1S/C24H26FN3O/c1-24(2)11-17-20(18(29)12-24)19(13-7-9-14(25)10-8-13)21-22(26)15-5-3-4-6-16(15)27-23(21)28-17/h7-10,19-20H,3-6,11-12H2,1-2H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50262341

(11-Amino-12-(4-methoxy-phenyl)-3,3-dimethyl-3,4,5,...)Show SMILES COc1ccc(cc1)C1C2C(=O)CC(C)(C)CC2=Nc2nc3CCCCc3c(N)c12 |c:19| Show InChI InChI=1S/C25H29N3O2/c1-25(2)12-18-21(19(29)13-25)20(14-8-10-15(30-3)11-9-14)22-23(26)16-6-4-5-7-17(16)27-24(22)28-18/h8-11,20-21H,4-7,12-13H2,1-3H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE by Ellman's method |
Bioorg Med Chem 16: 7759-69 (2008)
Article DOI: 10.1016/j.bmc.2008.07.005
BindingDB Entry DOI: 10.7270/Q2DZ096D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data