

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50344942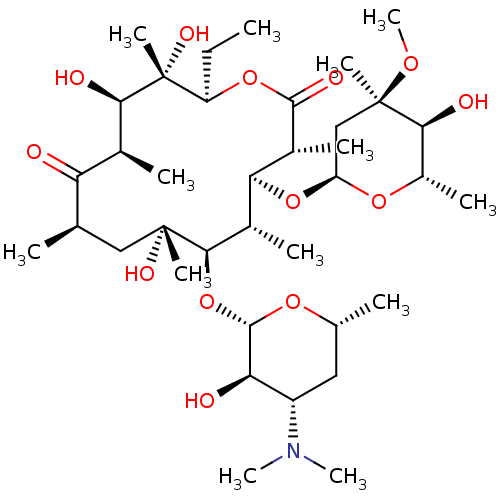 (CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50247157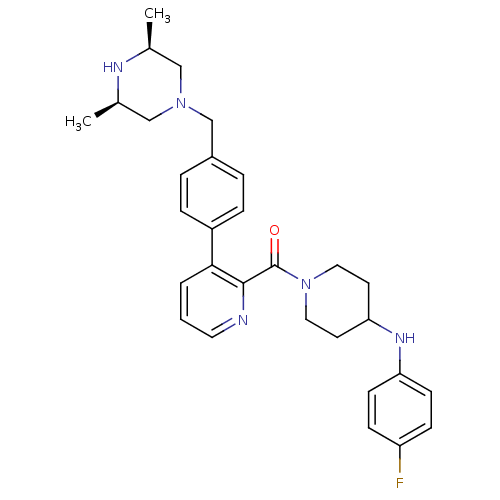 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50344942 (CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 0 to 5 mins by time dependent inhibition ass... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292984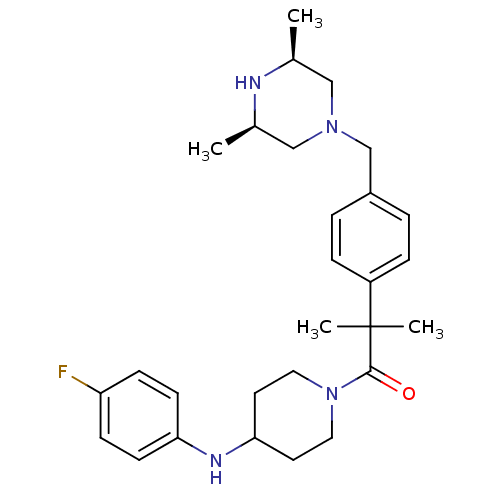 (1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292981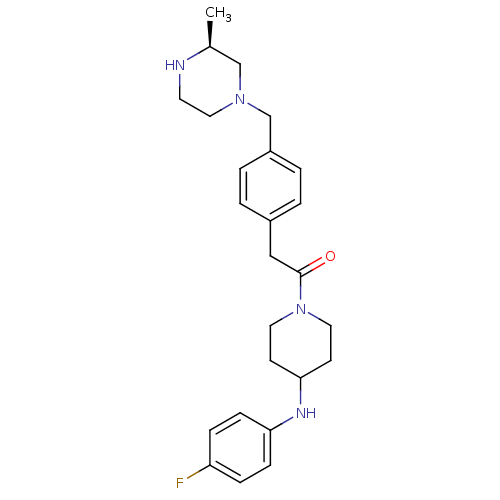 (CHEMBL489094 | N-(4-Fluorophenyl)-1-[(4-([(3S)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292983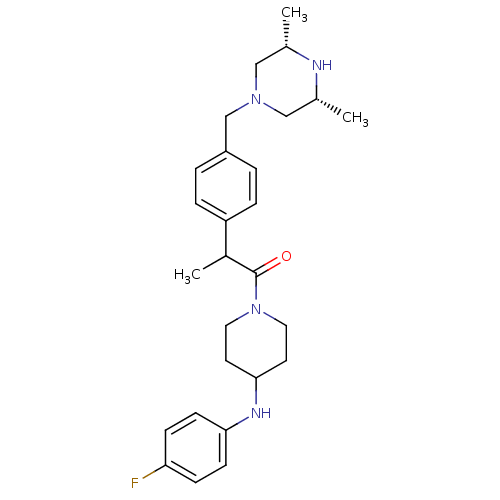 (1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292979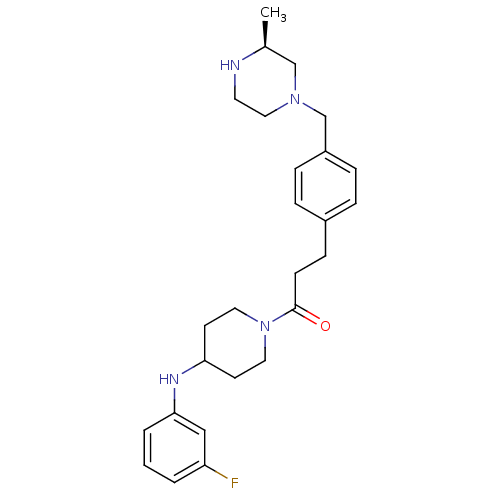 (CHEMBL474130 | N-(3-Fluorophenyl)-1-[3-(4-([(3S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292981 (CHEMBL489094 | N-(4-Fluorophenyl)-1-[(4-([(3S)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50292978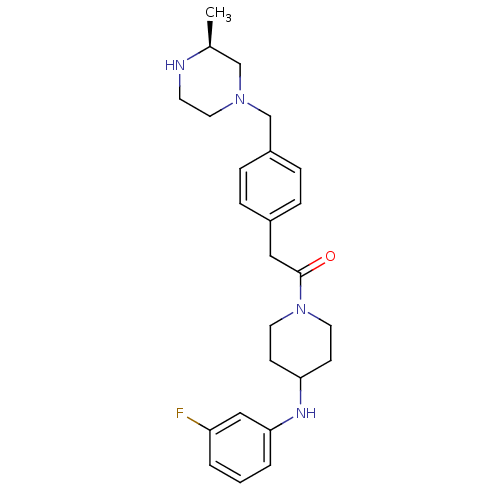 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human ERG | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292979 (CHEMBL474130 | N-(3-Fluorophenyl)-1-[3-(4-([(3S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 0 to 5 mins by time dependent inhibition ass... | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292982 (CHEMBL523933 | N-(3-Fluorophenyl)-1-[(4-([(3R)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human ERG | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292985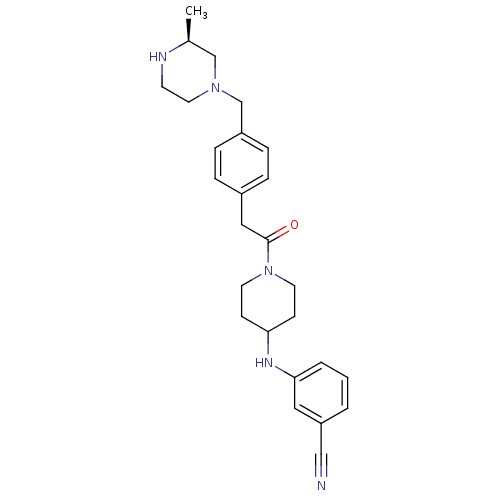 (3-((1-[(4-([(3S)-3-Methyl-1-piperazinyl]methyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292980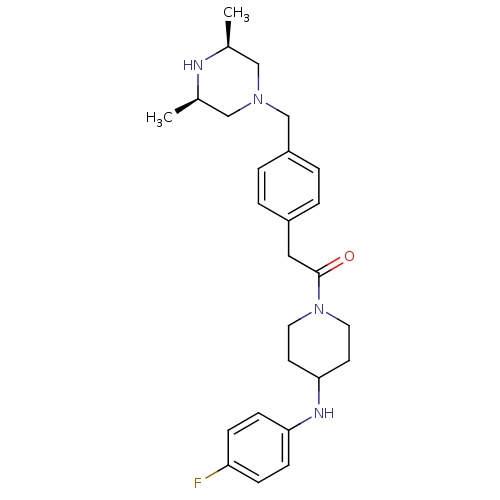 (1-[(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292985 (3-((1-[(4-([(3S)-3-Methyl-1-piperazinyl]methyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292980 (1-[(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292982 (CHEMBL523933 | N-(3-Fluorophenyl)-1-[(4-([(3R)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50412868 (CHEMBL474129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292980 (1-[(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292981 (CHEMBL489094 | N-(4-Fluorophenyl)-1-[(4-([(3S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292982 (CHEMBL523933 | N-(3-Fluorophenyl)-1-[(4-([(3R)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292983 (1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292984 (1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292985 (3-((1-[(4-([(3S)-3-Methyl-1-piperazinyl]methyl)phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM85389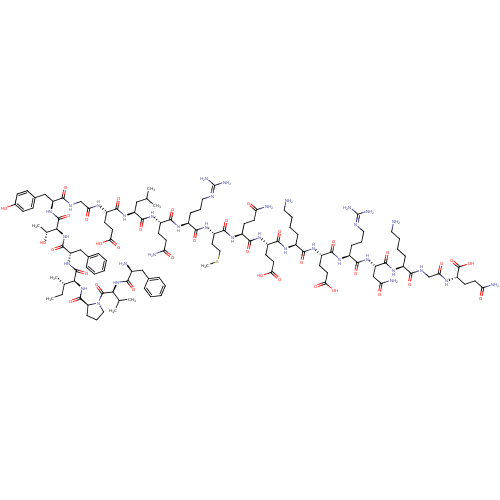 (CAS_52906-92-0 | Motilin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50344942 (CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50247157 ((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human Ghrelin receptor | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50292978 (CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human Ghrelin receptor | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50412867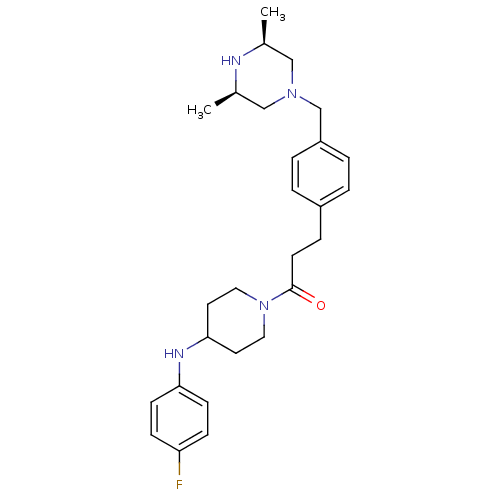 (CHEMBL514475) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50292979 (CHEMBL474130 | N-(3-Fluorophenyl)-1-[3-(4-([(3S)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human recombinant motilin receptor expressed in CHO cells assessed as increase in intracellular calcium by FLIPR assay | J Med Chem 52: 1180-9 (2009) Article DOI: 10.1021/jm801332q BindingDB Entry DOI: 10.7270/Q208666T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||