

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34143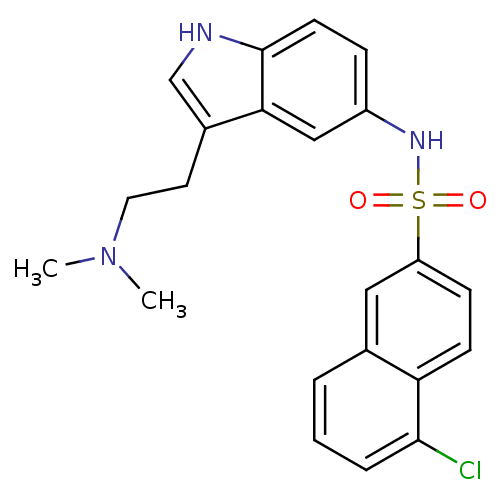 (CHEMBL175835 | E-6837) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34141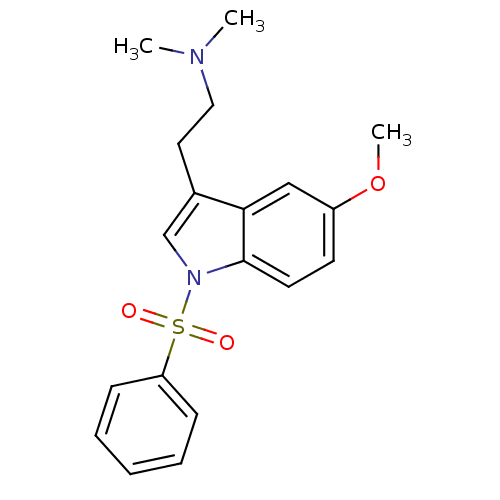 (CHEMBL76237 | MS-245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34152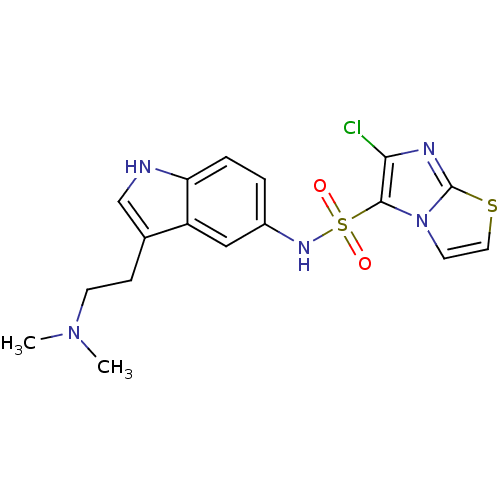 (CHEMBL362628 | E-6801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266553 (2-(1-benzyl-1H-inden-3-yl)-N,N-dimethylethanamine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50106250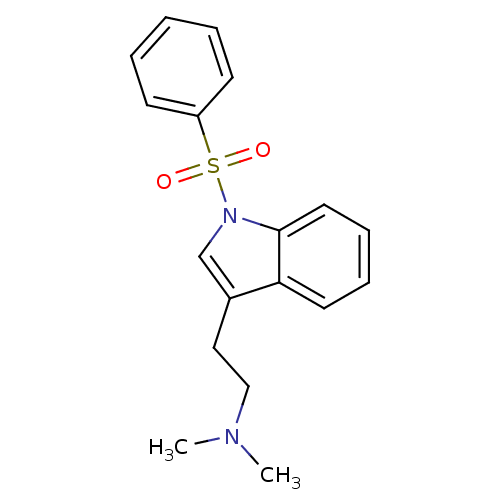 (CHEMBL93868 | N,N-dimethyl-2-(1-(phenylsulfonyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34153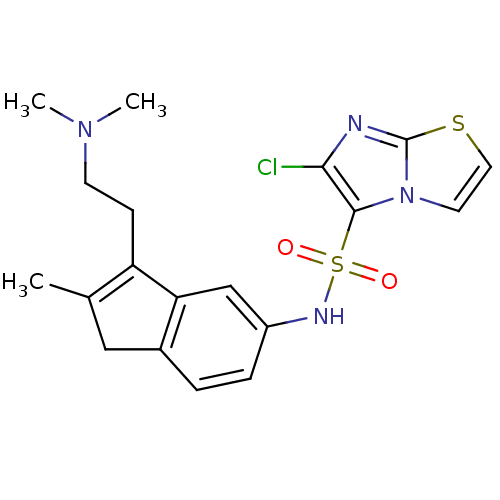 (CHEMBL476006 | indenylsulfonamide, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50164758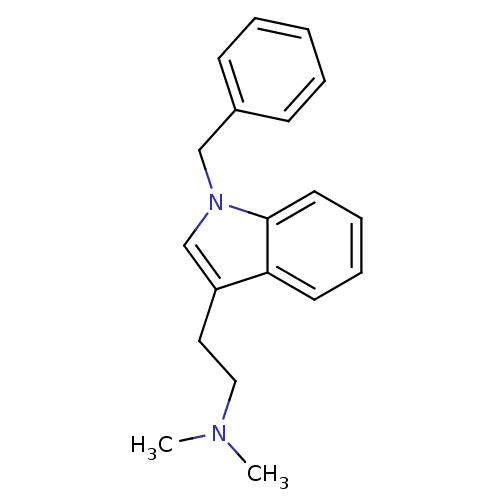 (2-(1-benzyl-1H-indol-3-yl)-N,N-dimethylethanamine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34154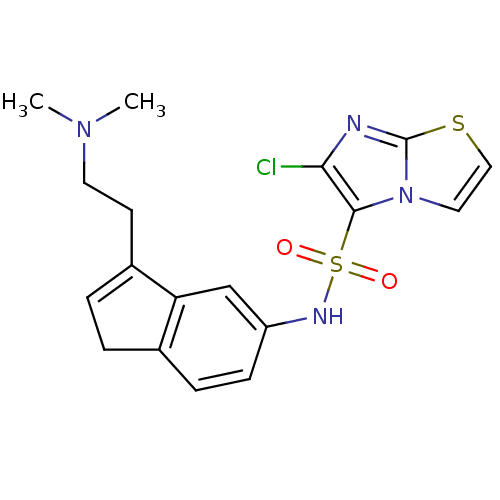 (CHEMBL515307 | indenylsulfonamide, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM28583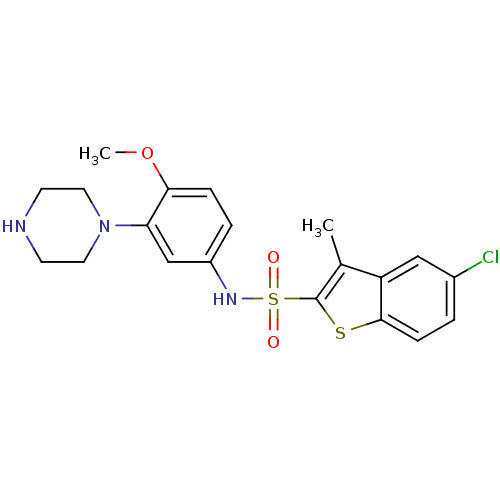 (5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34142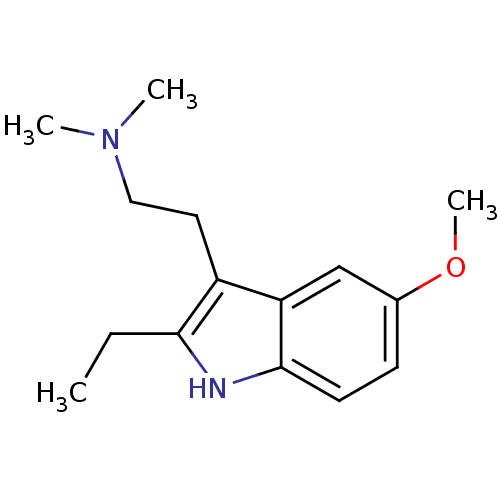 (2-ethyl-5-methoxy-N,N-dimethyltryptamine | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266617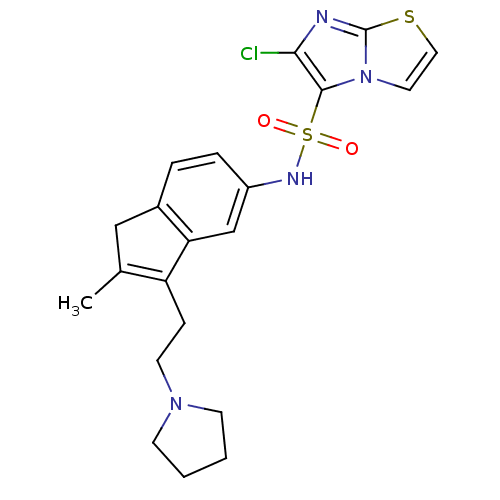 (6-Chloro-N-[2-methyl-3-(2-pyrrolidin-1-ylethyl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266576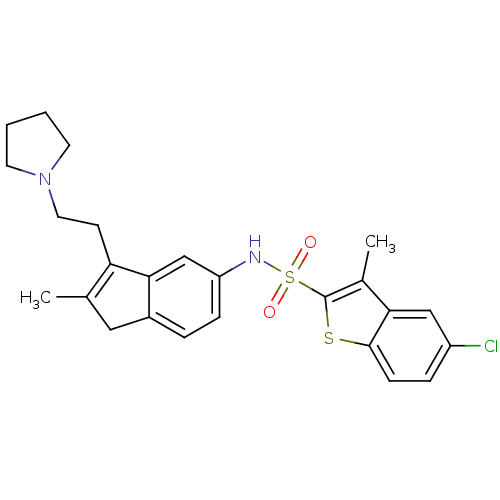 (5-chloro-3-methyl-N-(2-methyl-3-(2-(pyrrolidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266618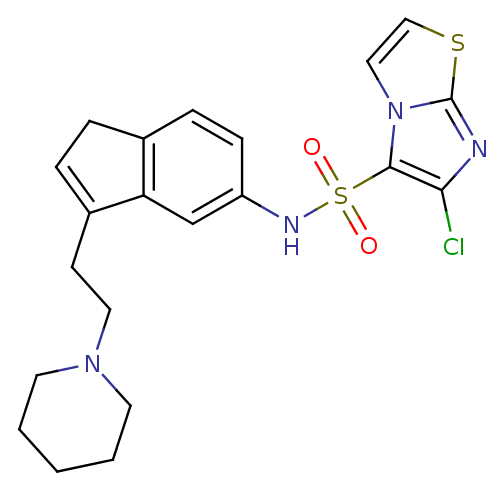 (6-Chloro-N-[3-(2-piperidin-1-ylethyl)-1H-inden-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50090528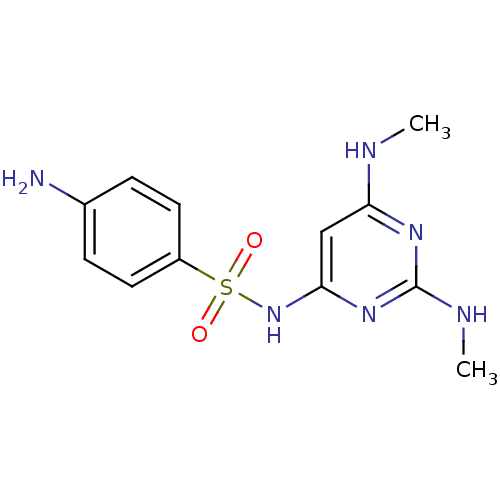 (4-Amino-N-(2,6-bis-dimethylamino-pyrimidin-4-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266555 (CHEMBL457570 | N-(3-(2-(dimethylamino)ethyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266578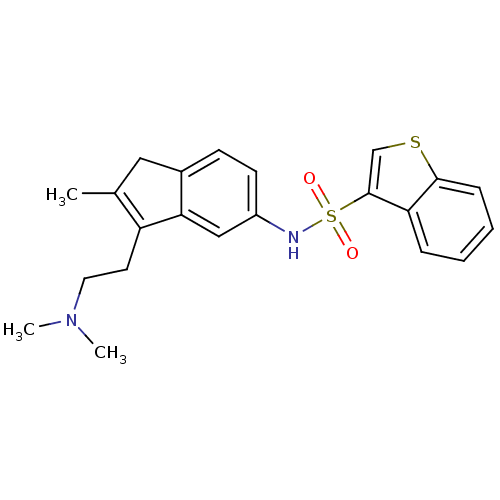 (CHEMBL477650 | N-{3-[2-(Dimethylamino)ethyl]-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266554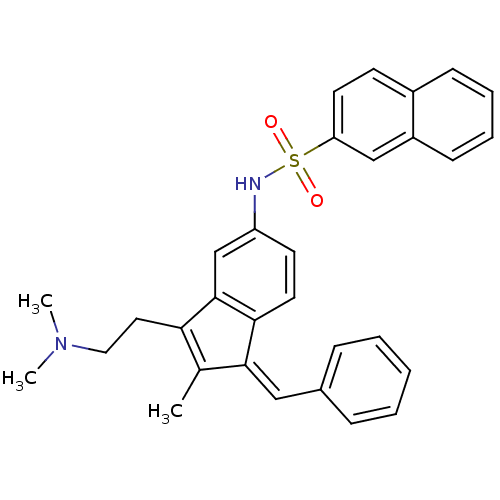 (CHEMBL457569 | N-(1-benzylidene-3-(2-(dimethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266577 (CHEMBL478896 | N-{3-[2-(Dimethylamino)ethyl]-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50266578 (CHEMBL477650 | N-{3-[2-(Dimethylamino)ethyl]-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human adrenergic alpha2A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM34153 (CHEMBL476006 | indenylsulfonamide, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM34154 (CHEMBL515307 | indenylsulfonamide, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50266639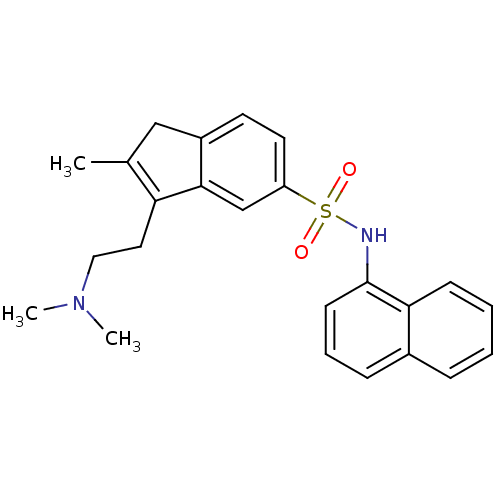 (3-(2-(dimethylamino)ethyl)-2-methyl-N-(naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM34153 (CHEMBL476006 | indenylsulfonamide, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human adrenergic alpha2A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM34154 (CHEMBL515307 | indenylsulfonamide, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human adrenergic alpha2A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50266555 (CHEMBL457570 | N-(3-(2-(dimethylamino)ethyl)-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50266555 (CHEMBL457570 | N-(3-(2-(dimethylamino)ethyl)-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human adrenergic alpha2A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM34154 (CHEMBL515307 | indenylsulfonamide, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT2C receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50266639 (3-(2-(dimethylamino)ethyl)-2-methyl-N-(naphthalen-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human adrenergic alpha2A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM34153 (CHEMBL476006 | indenylsulfonamide, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT2C receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50266578 (CHEMBL477650 | N-{3-[2-(Dimethylamino)ethyl]-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human 5HT1A receptor | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM34154 (CHEMBL515307 | indenylsulfonamide, 15) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human SERT | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM34153 (CHEMBL476006 | indenylsulfonamide, 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human SERT | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34153 (CHEMBL476006 | indenylsulfonamide, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Agonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as stimulation of cAMP level after 30 mins by HTRF assay Inhibition of ra... | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM34154 (CHEMBL515307 | indenylsulfonamide, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Agonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as stimulation of cAMP level after 30 mins by HTRF assay Inhibition of ra... | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266639 (3-(2-(dimethylamino)ethyl)-2-methyl-N-(naphthalen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Agonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as stimulation of cAMP level after 30 mins by HTRF assay Inhibition of ra... | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266578 (CHEMBL477650 | N-{3-[2-(Dimethylamino)ethyl]-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Agonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as stimulation of cAMP level after 30 mins by HTRF assay Inhibition of ra... | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50266555 (CHEMBL457570 | N-(3-(2-(dimethylamino)ethyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Agonist activity at human 5HT6 receptor expressed in HEK293F cells assessed as stimulation of cAMP level after 30 mins by HTRF assay Inhibition of ra... | J Med Chem 52: 675-87 (2009) Article DOI: 10.1021/jm8009469 BindingDB Entry DOI: 10.7270/Q2SN09V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||