

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50029031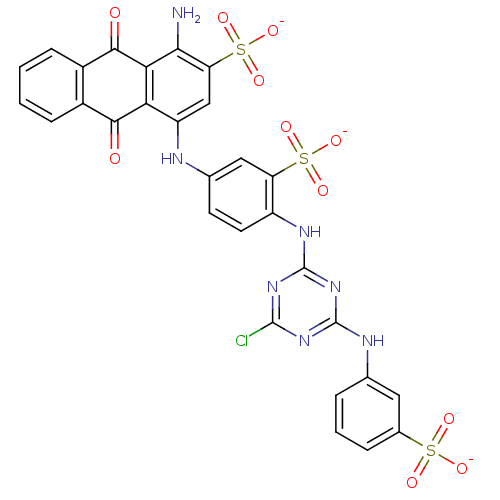 (1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as inhibition of UTP-ind... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50029031 (1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at N-terminal HA epitope-tagged wild type 4 human P2Y2 receptor expressed in human 1321N1 cells assessed as inhibition of UTP-ind... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50227023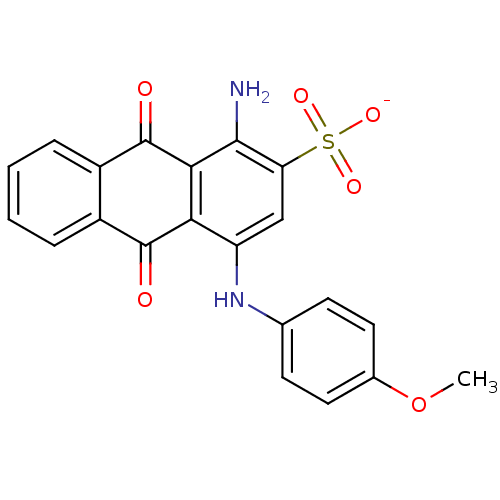 (1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at N-terminal HA epitope-tagged wild type 4 human P2Y2 receptor expressed in human 1321N1 cells assessed as inhibition of UTP-ind... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50227023 (1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as inhibition of UTP-ind... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118220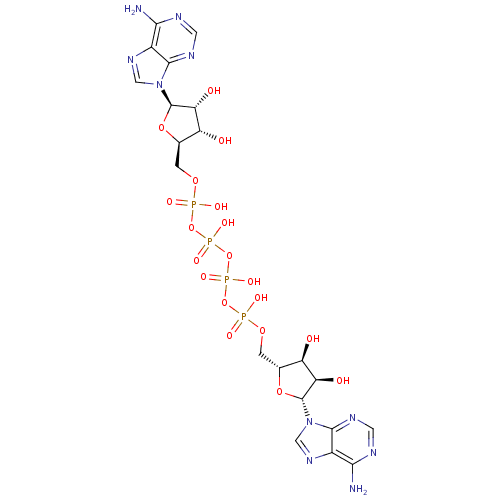 ((ppA)2 | A(5')p4(5')A | CHEMBL339385 | P(1),P(4)-b...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 145 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50366480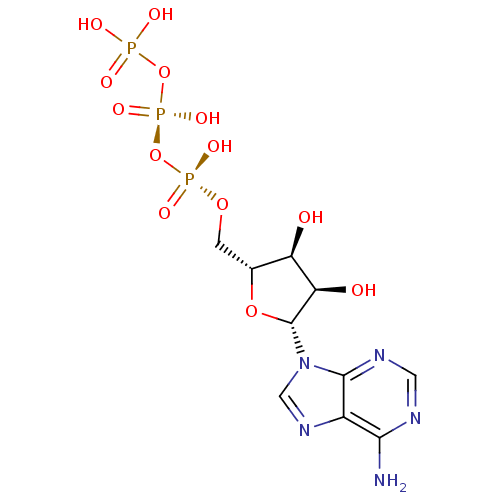 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 95.8 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 4 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 781 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 1 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 80.4 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205413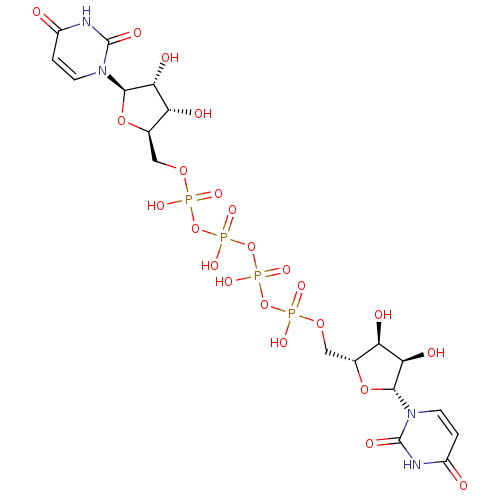 (CHEMBL221326 | P(1),P(4)-bis(uridin-5'-yl) tetraph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 112 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 4 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205413 (CHEMBL221326 | P(1),P(4)-bis(uridin-5'-yl) tetraph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 179 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 4 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118220 ((ppA)2 | A(5')p4(5')A | CHEMBL339385 | P(1),P(4)-b...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 167 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 4 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50227023 (1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80.4 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at N-terminal HA epitope-tagged wild type 3 human P2Y2 receptor expressed in human 1321N1 cells assessed as increase in intracellula... | J Med Chem 52: 2762-75 (2009) Article DOI: 10.1021/jm801442p BindingDB Entry DOI: 10.7270/Q2QC04FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||