

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265728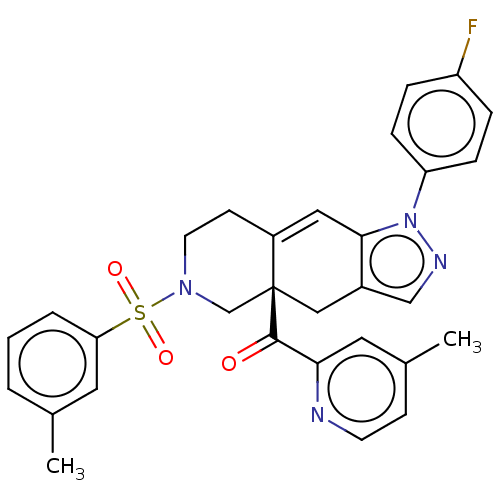 (CHEMBL4081121) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265798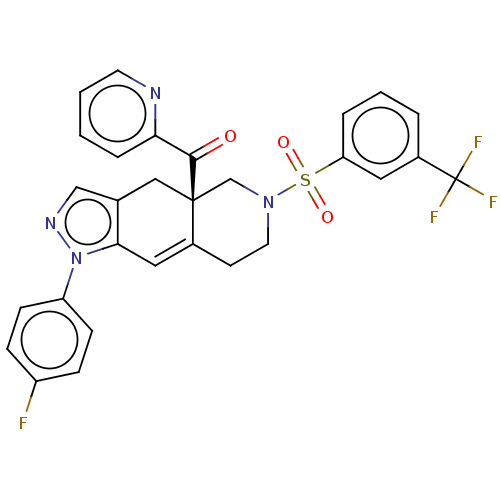 (CHEMBL3736358) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265797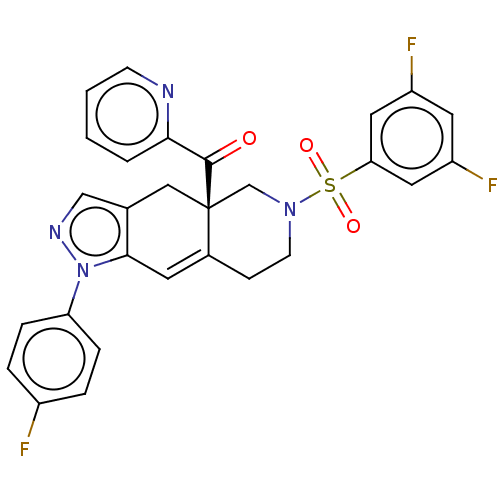 (CHEMBL4088286) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265796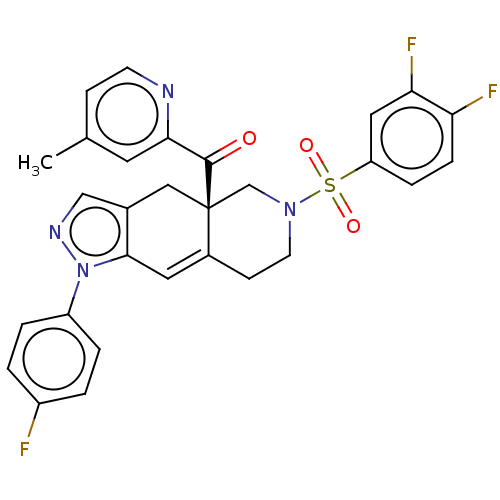 (CHEMBL4067017) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265729 (CHEMBL4105376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265801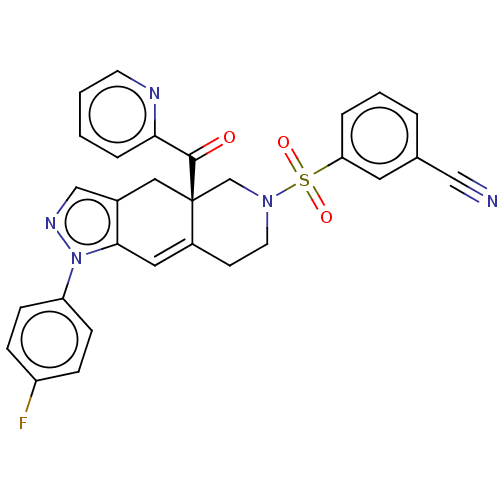 (CHEMBL3735851) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265803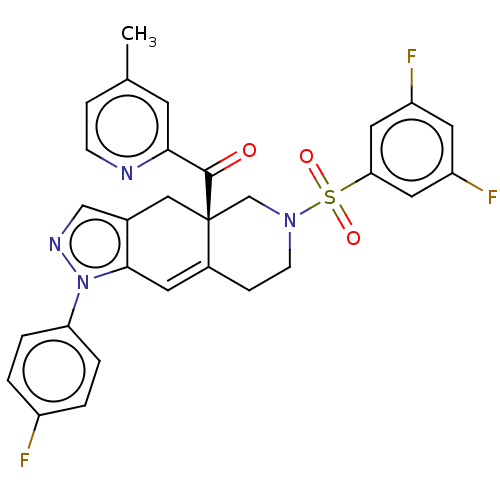 (CHEMBL4077976) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265804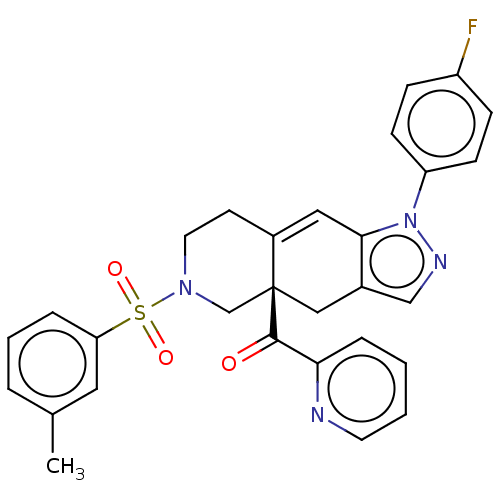 (CHEMBL3735124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265730 (CHEMBL4101325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265806 (CHEMBL4074627) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265802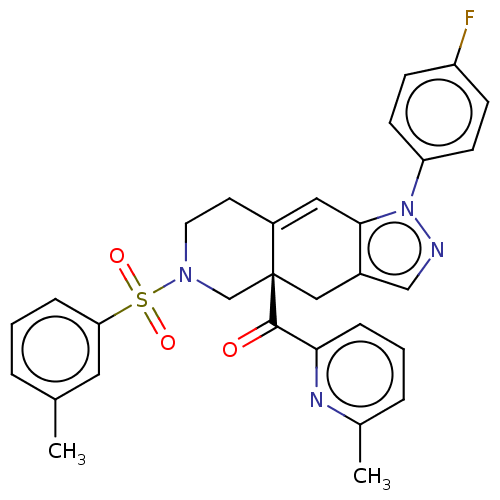 (CHEMBL4089006) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265799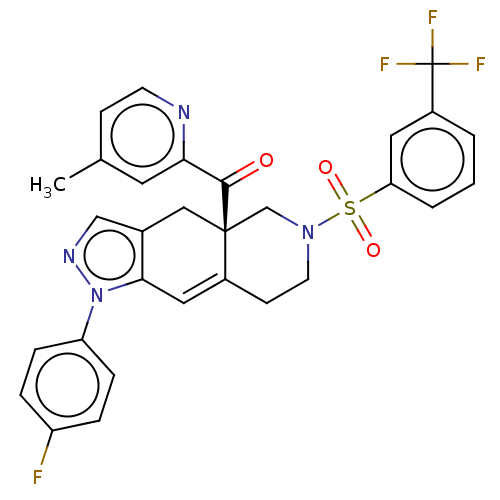 (CHEMBL4096930) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265795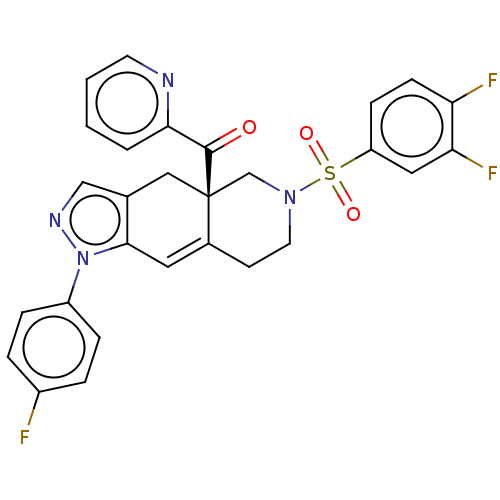 (CHEMBL3736000) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265735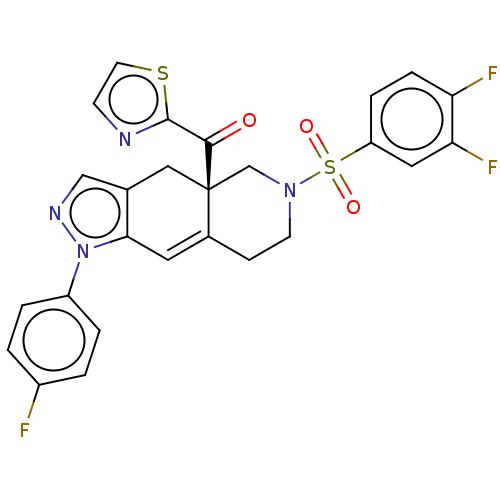 (CHEMBL4073649) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674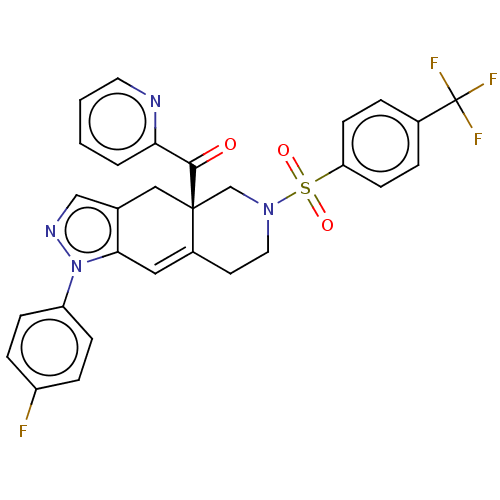 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265805 (CHEMBL3735218) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265807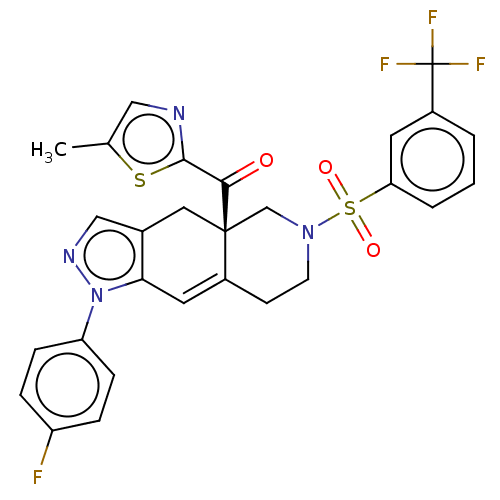 (CHEMBL4084014) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265731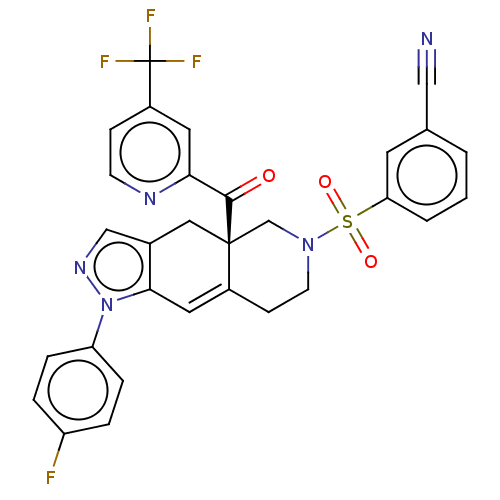 (CHEMBL4091787) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265762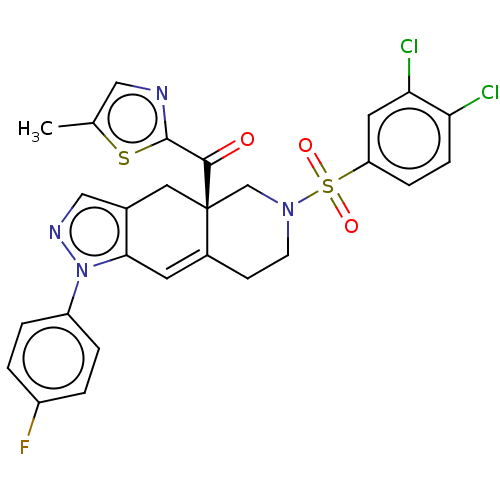 (CHEMBL4066669) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265800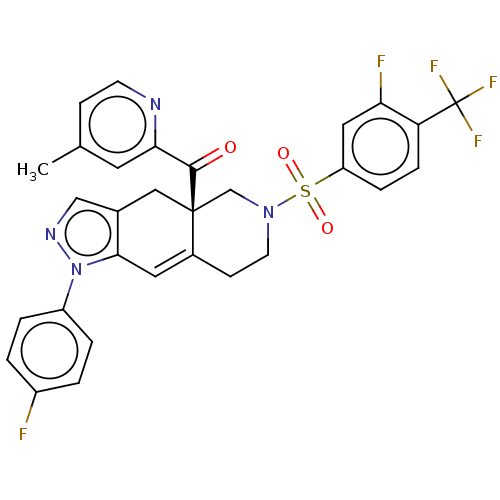 (CHEMBL4104661) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265732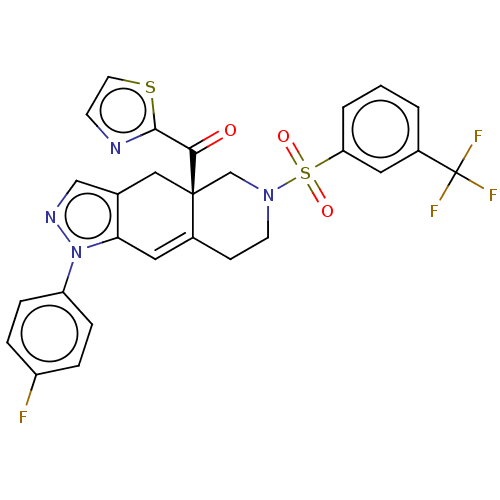 (CHEMBL4070919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265815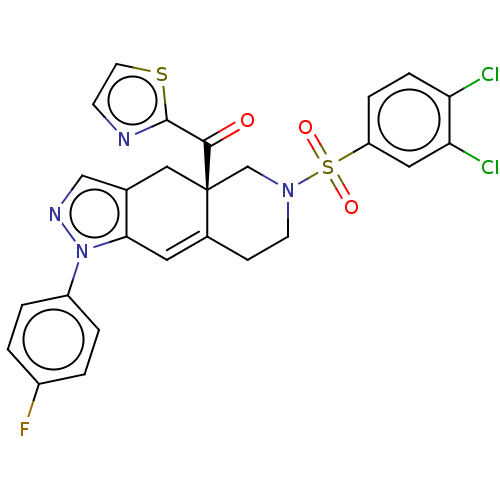 (CHEMBL4065064) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265734 (CHEMBL4062302) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265736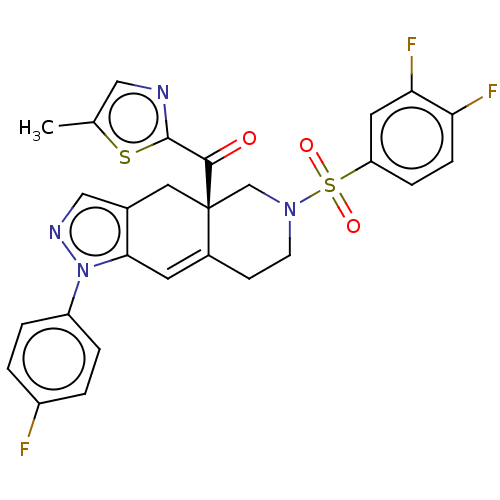 (CHEMBL4069516) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265806 (CHEMBL4074627) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265832 (CHEMBL4068844) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265728 (CHEMBL4081121) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265803 (CHEMBL4077976) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265730 (CHEMBL4101325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265830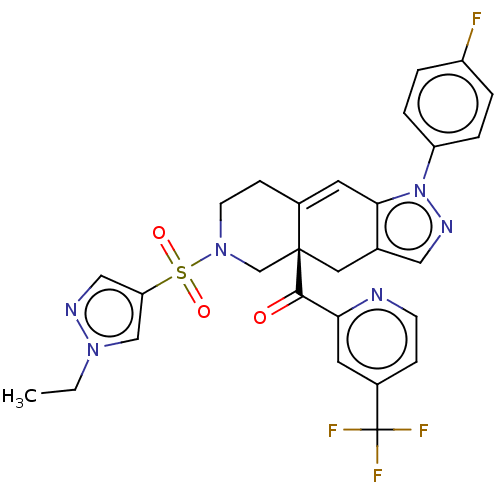 (CHEMBL4104804) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265731 (CHEMBL4091787) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265799 (CHEMBL4096930) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265796 (CHEMBL4067017) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265766 (CHEMBL4060190) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265804 (CHEMBL3735124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265673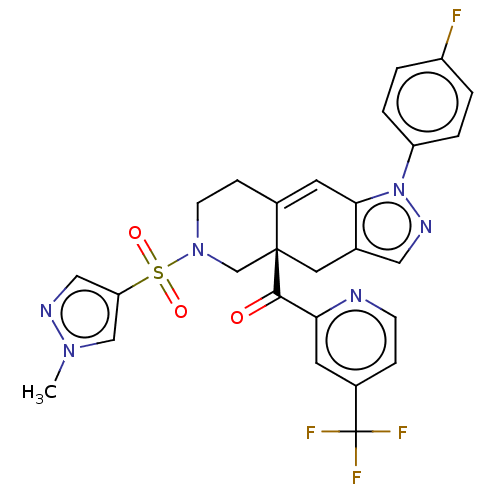 (CHEMBL4068611) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265736 (CHEMBL4069516) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265762 (CHEMBL4066669) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265795 (CHEMBL3736000) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265800 (CHEMBL4104661) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265729 (CHEMBL4105376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265831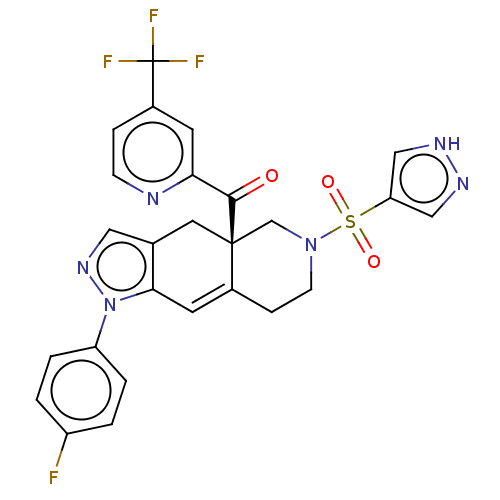 (CHEMBL4071548) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265797 (CHEMBL4088286) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265788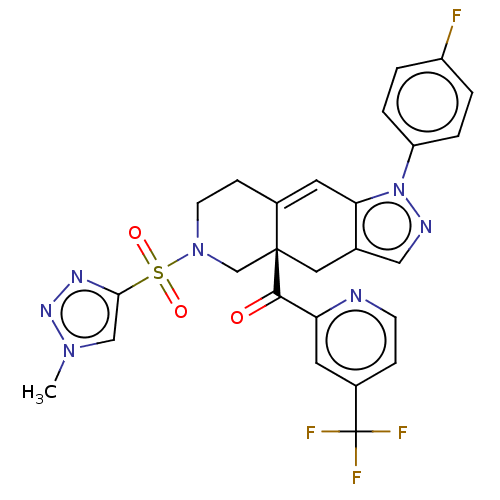 (CHEMBL4089940) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265807 (CHEMBL4084014) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265781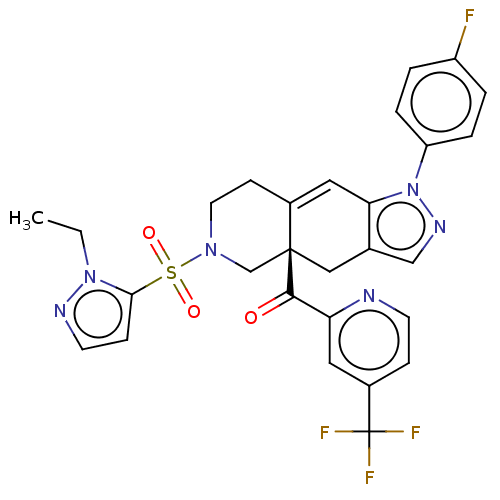 (CHEMBL4079638) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265805 (CHEMBL3735218) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265770 (CHEMBL4082072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |