 Found 12 hits Enz. Inhib. hit(s) with all data for entry = 50039111
Found 12 hits Enz. Inhib. hit(s) with all data for entry = 50039111 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074
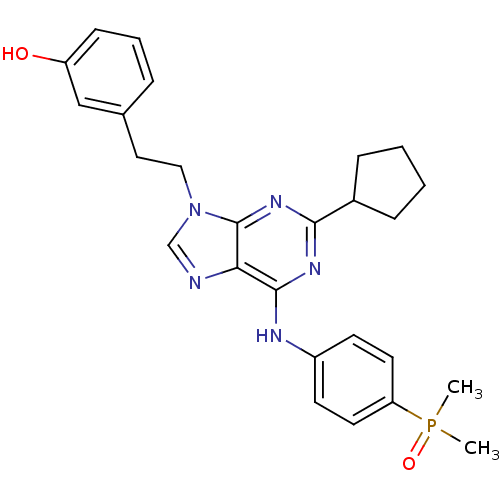
(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50236362

(CHEMBL429743 | N-(4-chlorophenyl)-6-(6,7-dimethoxy...)Show SMILES COc1cc2nccc(Oc3ccc4c(cccc4c3)C(=O)Nc3ccc(Cl)cc3)c2cc1OC Show InChI InChI=1S/C28H21ClN2O4/c1-33-26-15-23-24(16-27(26)34-2)30-13-12-25(23)35-20-10-11-21-17(14-20)4-3-5-22(21)28(32)31-19-8-6-18(29)7-9-19/h3-16H,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50236856
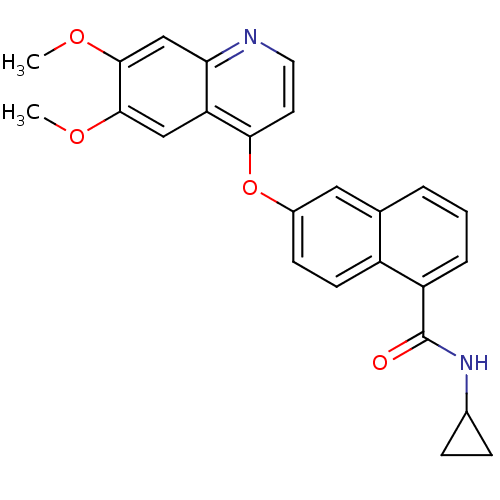
(CHEMBL272198 | N-cyclopropyl-6-(6,7-dimethoxyquino...)Show SMILES COc1cc2nccc(Oc3ccc4c(cccc4c3)C(=O)NC3CC3)c2cc1OC Show InChI InChI=1S/C25H22N2O4/c1-29-23-13-20-21(14-24(23)30-2)26-11-10-22(20)31-17-8-9-18-15(12-17)4-3-5-19(18)25(28)27-16-6-7-16/h3-5,8-14,16H,6-7H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50204694
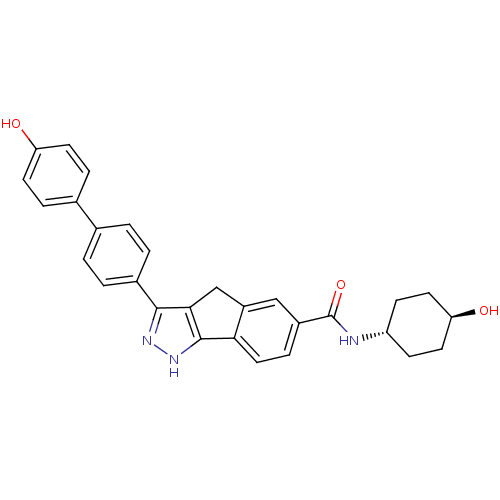
(3-(4'-HYDROXYBIPHENYL-4-YL)-N-(4-HYDROXYCYCLOHEXYL...)Show SMILES O[C@H]1CC[C@@H](CC1)NC(=O)c1ccc-2c(Cc3c(n[nH]c-23)-c2ccc(cc2)-c2ccc(O)cc2)c1 |wU:4.7,wD:1.0,(19.77,-1.9,;21.02,-1.01,;22.42,-1.66,;23.69,-.77,;23.53,.76,;22.14,1.41,;20.89,.52,;24.79,1.65,;26.19,1.01,;26.33,-.53,;27.44,1.9,;27.3,3.44,;28.55,4.33,;29.95,3.7,;30.1,2.15,;31.61,1.81,;32.4,3.14,;33.83,3.77,;33.68,5.31,;32.16,5.65,;31.37,4.31,;35.16,2.99,;35.15,1.44,;36.46,.66,;37.81,1.42,;37.82,2.96,;36.49,3.74,;39.13,.64,;39.12,-.9,;40.44,-1.67,;41.78,-.91,;43.11,-1.69,;41.78,.63,;40.46,1.4,;28.85,1.26,)| Show InChI InChI=1S/C29H27N3O3/c33-23-10-5-18(6-11-23)17-1-3-19(4-2-17)27-26-16-21-15-20(7-14-25(21)28(26)32-31-27)29(35)30-22-8-12-24(34)13-9-22/h1-7,10-11,14-15,22,24,33-34H,8-9,12-13,16H2,(H,30,35)(H,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
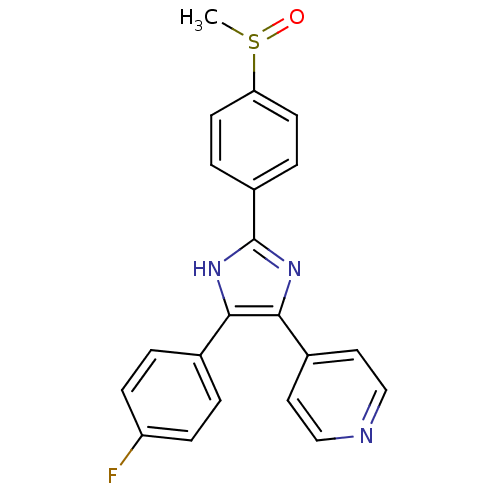
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MAPK14 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM27216

((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2cccc(Cl)c2)c2ncn(C(C)C)c2n1 Show InChI InChI=1S/C19H25ClN6O/c1-11(2)15(9-27)23-19-24-17(22-14-7-5-6-13(20)8-14)16-18(25-19)26(10-21-16)12(3)4/h5-8,10-12,15,27H,9H2,1-4H3,(H2,22,23,24,25)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM27216

((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2cccc(Cl)c2)c2ncn(C(C)C)c2n1 Show InChI InChI=1S/C19H25ClN6O/c1-11(2)15(9-27)23-19-24-17(22-14-7-5-6-13(20)8-14)16-18(25-19)26(10-21-16)12(3)4/h5-8,10-12,15,27H,9H2,1-4H3,(H2,22,23,24,25)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50314075

(1-(5-(2-(6-(1H-1,2,4-triazol-3-ylamino)pyrimidin-4...)Show SMILES Cc1cc(NC(=O)Nc2ncc(CCNc3cc(Nc4nnc[nH]4)ncn3)s2)c(o1)C(F)(F)F Show InChI InChI=1S/C18H17F3N10O2S/c1-9-4-11(14(33-9)18(19,20)21)28-16(32)30-17-23-6-10(34-17)2-3-22-12-5-13(25-7-24-12)29-15-26-8-27-31-15/h4-8H,2-3H2,1H3,(H2,23,28,30,32)(H3,22,24,25,26,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50204694

(3-(4'-HYDROXYBIPHENYL-4-YL)-N-(4-HYDROXYCYCLOHEXYL...)Show SMILES O[C@H]1CC[C@@H](CC1)NC(=O)c1ccc-2c(Cc3c(n[nH]c-23)-c2ccc(cc2)-c2ccc(O)cc2)c1 |wU:4.7,wD:1.0,(19.77,-1.9,;21.02,-1.01,;22.42,-1.66,;23.69,-.77,;23.53,.76,;22.14,1.41,;20.89,.52,;24.79,1.65,;26.19,1.01,;26.33,-.53,;27.44,1.9,;27.3,3.44,;28.55,4.33,;29.95,3.7,;30.1,2.15,;31.61,1.81,;32.4,3.14,;33.83,3.77,;33.68,5.31,;32.16,5.65,;31.37,4.31,;35.16,2.99,;35.15,1.44,;36.46,.66,;37.81,1.42,;37.82,2.96,;36.49,3.74,;39.13,.64,;39.12,-.9,;40.44,-1.67,;41.78,-.91,;43.11,-1.69,;41.78,.63,;40.46,1.4,;28.85,1.26,)| Show InChI InChI=1S/C29H27N3O3/c33-23-10-5-18(6-11-23)17-1-3-19(4-2-17)27-26-16-21-15-20(7-14-25(21)28(26)32-31-27)29(35)30-22-8-12-24(34)13-9-22/h1-7,10-11,14-15,22,24,33-34H,8-9,12-13,16H2,(H,30,35)(H,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SGK |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50204694

(3-(4'-HYDROXYBIPHENYL-4-YL)-N-(4-HYDROXYCYCLOHEXYL...)Show SMILES O[C@H]1CC[C@@H](CC1)NC(=O)c1ccc-2c(Cc3c(n[nH]c-23)-c2ccc(cc2)-c2ccc(O)cc2)c1 |wU:4.7,wD:1.0,(19.77,-1.9,;21.02,-1.01,;22.42,-1.66,;23.69,-.77,;23.53,.76,;22.14,1.41,;20.89,.52,;24.79,1.65,;26.19,1.01,;26.33,-.53,;27.44,1.9,;27.3,3.44,;28.55,4.33,;29.95,3.7,;30.1,2.15,;31.61,1.81,;32.4,3.14,;33.83,3.77,;33.68,5.31,;32.16,5.65,;31.37,4.31,;35.16,2.99,;35.15,1.44,;36.46,.66,;37.81,1.42,;37.82,2.96,;36.49,3.74,;39.13,.64,;39.12,-.9,;40.44,-1.67,;41.78,-.91,;43.11,-1.69,;41.78,.63,;40.46,1.4,;28.85,1.26,)| Show InChI InChI=1S/C29H27N3O3/c33-23-10-5-18(6-11-23)17-1-3-19(4-2-17)27-26-16-21-15-20(7-14-25(21)28(26)32-31-27)29(35)30-22-8-12-24(34)13-9-22/h1-7,10-11,14-15,22,24,33-34H,8-9,12-13,16H2,(H,30,35)(H,31,32)/t22-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50204694

(3-(4'-HYDROXYBIPHENYL-4-YL)-N-(4-HYDROXYCYCLOHEXYL...)Show SMILES O[C@H]1CC[C@@H](CC1)NC(=O)c1ccc-2c(Cc3c(n[nH]c-23)-c2ccc(cc2)-c2ccc(O)cc2)c1 |wU:4.7,wD:1.0,(19.77,-1.9,;21.02,-1.01,;22.42,-1.66,;23.69,-.77,;23.53,.76,;22.14,1.41,;20.89,.52,;24.79,1.65,;26.19,1.01,;26.33,-.53,;27.44,1.9,;27.3,3.44,;28.55,4.33,;29.95,3.7,;30.1,2.15,;31.61,1.81,;32.4,3.14,;33.83,3.77,;33.68,5.31,;32.16,5.65,;31.37,4.31,;35.16,2.99,;35.15,1.44,;36.46,.66,;37.81,1.42,;37.82,2.96,;36.49,3.74,;39.13,.64,;39.12,-.9,;40.44,-1.67,;41.78,-.91,;43.11,-1.69,;41.78,.63,;40.46,1.4,;28.85,1.26,)| Show InChI InChI=1S/C29H27N3O3/c33-23-10-5-18(6-11-23)17-1-3-19(4-2-17)27-26-16-21-15-20(7-14-25(21)28(26)32-31-27)29(35)30-22-8-12-24(34)13-9-22/h1-7,10-11,14-15,22,24,33-34H,8-9,12-13,16H2,(H,30,35)(H,31,32)/t22-,24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of aurora 1 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50314074

(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data