

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50317074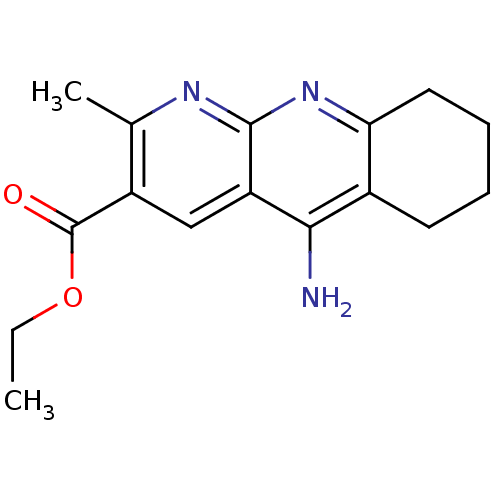 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE (unknown orign) by Lineweaver-Burke plot | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317074 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50317074 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318705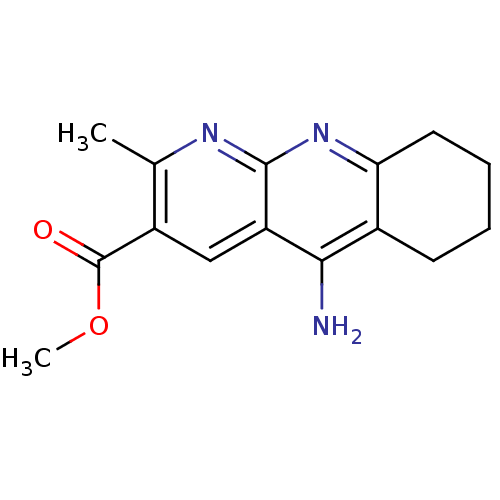 (CHEMBL1086184 | Methyl 5-Amino-2-methyl-6,7,8,9-te...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318705 (CHEMBL1086184 | Methyl 5-Amino-2-methyl-6,7,8,9-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318703 (CHEMBL1083972 | Methyl 5-Amino-2-methyl-7,8-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318703 (CHEMBL1083972 | Methyl 5-Amino-2-methyl-7,8-dihydr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318708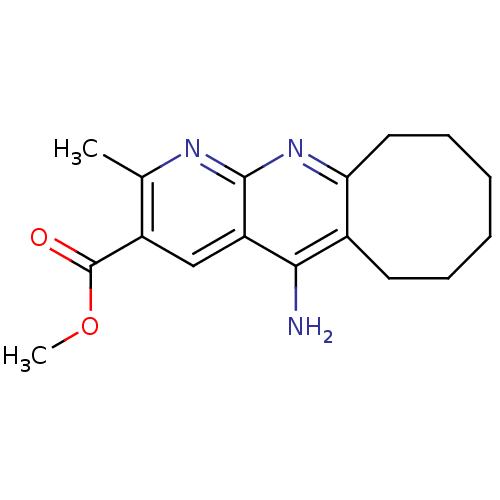 (CHEMBL1084517 | Methyl 5-Amino-2-methyl-6,7,8,9,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318708 (CHEMBL1084517 | Methyl 5-Amino-2-methyl-6,7,8,9,10...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318706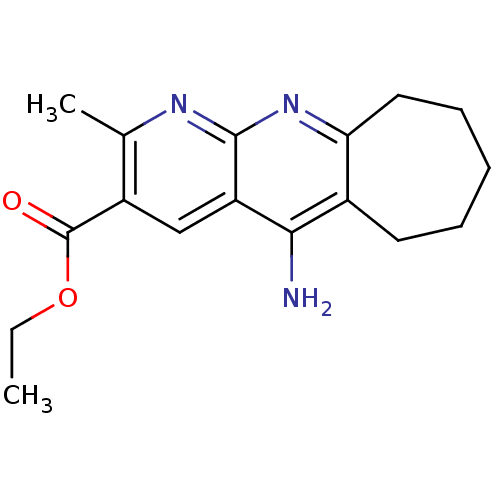 (CHEMBL1083973 | Ethyl 5-Amino-2-methyl-7,8,9,10-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318706 (CHEMBL1083973 | Ethyl 5-Amino-2-methyl-7,8,9,10-te...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318702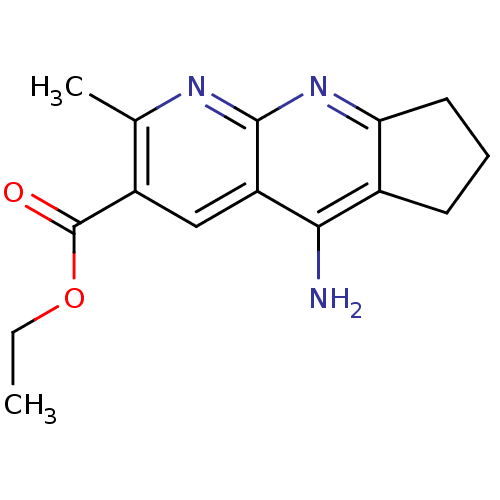 (CHEMBL1084518 | Ethyl 5-Amino-2-methyl-7,8-dihydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318702 (CHEMBL1084518 | Ethyl 5-Amino-2-methyl-7,8-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318707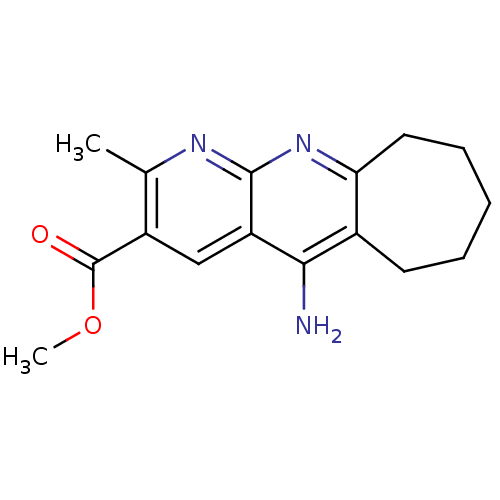 (CHEMBL1084250 | Methyl 5-Amino-2-methyl-7,8,9,10-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318707 (CHEMBL1084250 | Methyl 5-Amino-2-methyl-7,8,9,10-t...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50318704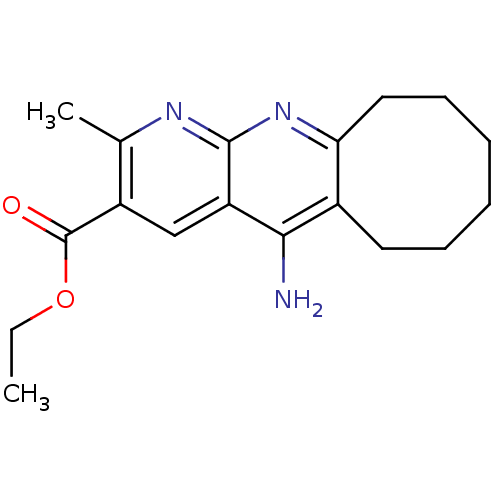 (CHEMBL1084519 | Ethyl 5-Amino-2-methyl-6,7,8,9,10,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Displacement of propidium iodide from AChE in bovine erythrocytes after 15 mins by fluorescence plate reader | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50318704 (CHEMBL1084519 | Ethyl 5-Amino-2-methyl-6,7,8,9,10,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318707 (CHEMBL1084250 | Methyl 5-Amino-2-methyl-7,8,9,10-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50317074 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50241350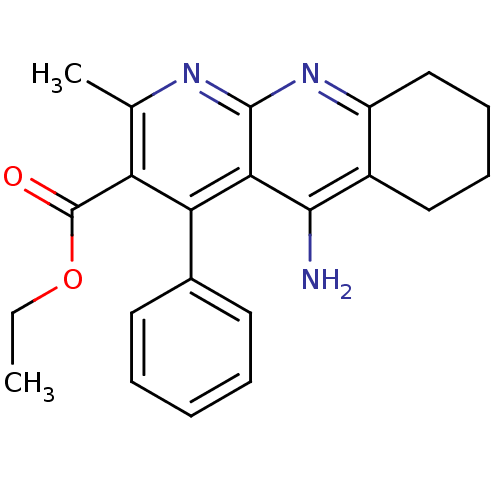 (5-Amino-2-methyl-4-phenyl-6,7,8,9-tetrahydro-benzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318705 (CHEMBL1086184 | Methyl 5-Amino-2-methyl-6,7,8,9-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318708 (CHEMBL1084517 | Methyl 5-Amino-2-methyl-6,7,8,9,10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318704 (CHEMBL1084519 | Ethyl 5-Amino-2-methyl-6,7,8,9,10,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318703 (CHEMBL1083972 | Methyl 5-Amino-2-methyl-7,8-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318707 (CHEMBL1084250 | Methyl 5-Amino-2-methyl-7,8,9,10-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318706 (CHEMBL1083973 | Ethyl 5-Amino-2-methyl-7,8,9,10-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318706 (CHEMBL1083973 | Ethyl 5-Amino-2-methyl-7,8,9,10-te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50318702 (CHEMBL1084518 | Ethyl 5-Amino-2-methyl-7,8-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318705 (CHEMBL1086184 | Methyl 5-Amino-2-methyl-6,7,8,9-te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318708 (CHEMBL1084517 | Methyl 5-Amino-2-methyl-6,7,8,9,10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318703 (CHEMBL1083972 | Methyl 5-Amino-2-methyl-7,8-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50241350 (5-Amino-2-methyl-4-phenyl-6,7,8,9-tetrahydro-benzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318704 (CHEMBL1084519 | Ethyl 5-Amino-2-methyl-6,7,8,9,10,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317074 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50318702 (CHEMBL1084518 | Ethyl 5-Amino-2-methyl-7,8-dihydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||