 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50039329
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50039329 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334300
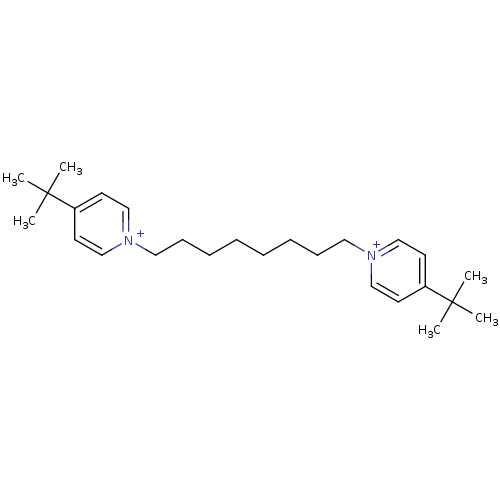
(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8958
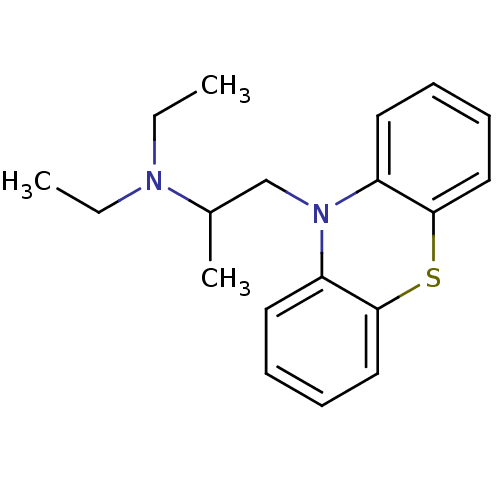
(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.21E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50304127

(1,12-Bis-(4-tert-butylpyridinium)dodecane dibromid...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H50N2/c1-29(2,3)27-17-23-31(24-18-27)21-15-13-11-9-7-8-10-12-14-16-22-32-25-19-28(20-26-32)30(4,5)6/h17-20,23-26H,7-16,21-22H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334301

(1,10-bis(4-tert.butylpyridinium)-dec-1,10-diyl dib...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C28H46N2/c1-27(2,3)25-15-21-29(22-16-25)19-13-11-9-7-8-10-12-14-20-30-23-17-26(18-24-30)28(4,5)6/h15-18,21-24H,7-14,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334302

(1,7-bis(4-tert.butylpyridinium)-hept-1,7-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C25H40N2/c1-24(2,3)22-12-18-26(19-13-22)16-10-8-7-9-11-17-27-20-14-23(15-21-27)25(4,5)6/h12-15,18-21H,7-11,16-17H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50304127

(1,12-Bis-(4-tert-butylpyridinium)dodecane dibromid...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C30H50N2/c1-29(2,3)27-17-23-31(24-18-27)21-15-13-11-9-7-8-10-12-14-16-22-32-25-19-28(20-26-32)30(4,5)6/h17-20,23-26H,7-16,21-22H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334304

(1,11-bis(4-tert.butylpyridinium)-undec-1,11-diyl d...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C29H48N2/c1-28(2,3)26-16-22-30(23-17-26)20-14-12-10-8-7-9-11-13-15-21-31-24-18-27(19-25-31)29(4,5)6/h16-19,22-25H,7-15,20-21H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334305
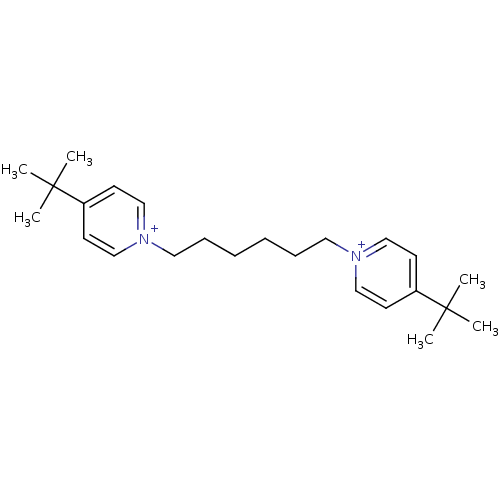
(1,6-bis(4-tert.butylpyridinium)-hex-1,6-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C24H38N2/c1-23(2,3)21-11-17-25(18-12-21)15-9-7-8-10-16-26-19-13-22(14-20-26)24(4,5)6/h11-14,17-20H,7-10,15-16H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334306

(1,9-bis(4-tert.butylpyridinium)-non-1,9-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H44N2/c1-26(2,3)24-14-20-28(21-15-24)18-12-10-8-7-9-11-13-19-29-22-16-25(17-23-29)27(4,5)6/h14-17,20-23H,7-13,18-19H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334301

(1,10-bis(4-tert.butylpyridinium)-dec-1,10-diyl dib...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C28H46N2/c1-27(2,3)25-15-21-29(22-16-25)19-13-11-9-7-8-10-12-14-20-30-23-17-26(18-24-30)28(4,5)6/h15-18,21-24H,7-14,19-20H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334304

(1,11-bis(4-tert.butylpyridinium)-undec-1,11-diyl d...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C29H48N2/c1-28(2,3)26-16-22-30(23-17-26)20-14-12-10-8-7-9-11-13-15-21-31-24-18-27(19-25-31)29(4,5)6/h16-19,22-25H,7-15,20-21H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334306

(1,9-bis(4-tert.butylpyridinium)-non-1,9-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C27H44N2/c1-26(2,3)24-14-20-28(21-15-24)18-12-10-8-7-9-11-13-19-29-22-16-25(17-23-29)27(4,5)6/h14-17,20-23H,7-13,18-19H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334307
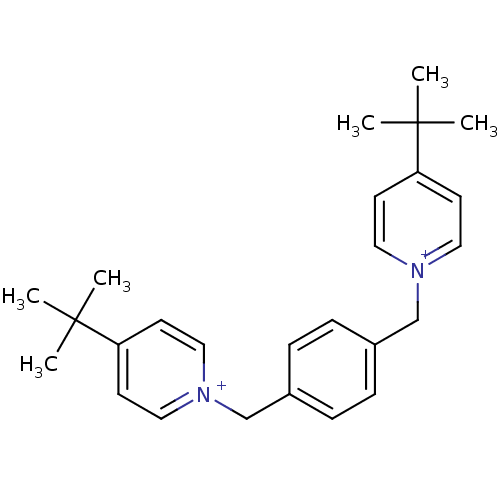
(1,1'-bis(4-tert.butylpyridinium)-1,4-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2ccc(C[n+]3ccc(cc3)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-11-15-27(16-12-23)19-21-7-9-22(10-8-21)20-28-17-13-24(14-18-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334305

(1,6-bis(4-tert.butylpyridinium)-hex-1,6-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C24H38N2/c1-23(2,3)21-11-17-25(18-12-21)15-9-7-8-10-16-26-19-13-22(14-20-26)24(4,5)6/h11-14,17-20H,7-10,15-16H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334302

(1,7-bis(4-tert.butylpyridinium)-hept-1,7-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C25H40N2/c1-24(2,3)22-12-18-26(19-13-22)16-10-8-7-9-11-17-27-20-14-23(15-21-27)25(4,5)6/h12-15,18-21H,7-11,16-17H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334308

(1,1'-bis(4-tert.butylpyridinium)-1,3-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2cccc(C[n+]3ccc(cc3)C(C)(C)C)c2)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-10-14-27(15-11-23)19-21-8-7-9-22(18-21)20-28-16-12-24(13-17-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334307

(1,1'-bis(4-tert.butylpyridinium)-1,4-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2ccc(C[n+]3ccc(cc3)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-11-15-27(16-12-23)19-21-7-9-22(10-8-21)20-28-17-13-24(14-18-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8958

(10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...)Show InChI InChI=1S/C19H24N2S/c1-4-20(5-2)15(3)14-21-16-10-6-8-12-18(16)22-19-13-9-7-11-17(19)21/h6-13,15H,4-5,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334308

(1,1'-bis(4-tert.butylpyridinium)-1,3-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2cccc(C[n+]3ccc(cc3)C(C)(C)C)c2)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-10-14-27(15-11-23)19-21-8-7-9-22(18-21)20-28-16-12-24(13-17-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334310

(1,4-bis(4-tert.butylpyridinium)-but-(2Z)-ene-1,4-d...)Show SMILES CC(C)(C)c1cc[n+](C\C=C/C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H32N2/c1-21(2,3)19-9-15-23(16-10-19)13-7-8-14-24-17-11-20(12-18-24)22(4,5)6/h7-12,15-18H,13-14H2,1-6H3/q+2/b8-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334311

(1,1'-bis(4-tert.butylpyridinium)-1,2-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2ccccc2C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-11-15-27(16-12-23)19-21-9-7-8-10-22(21)20-28-17-13-24(14-18-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334312

(1,4-bis(4-tert.butylpyridinium)-but-(2E)-ene-1,4-d...)Show SMILES CC(C)(C)c1cc[n+](C\C=C\C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H32N2/c1-21(2,3)19-9-15-23(16-10-19)13-7-8-14-24-17-11-20(12-18-24)22(4,5)6/h7-12,15-18H,13-14H2,1-6H3/q+2/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334313
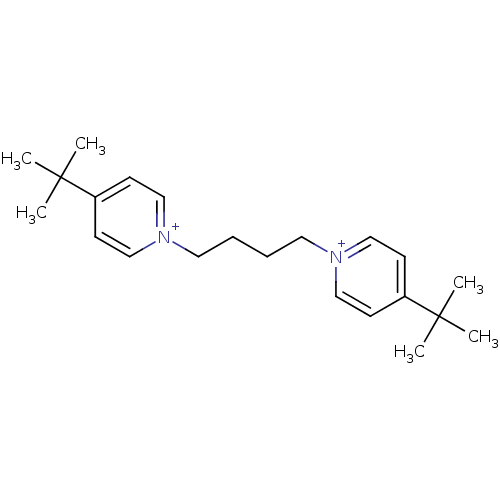
(1,4-bis(4-tert.butylpyridinium)-but-1,4-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H34N2/c1-21(2,3)19-9-15-23(16-10-19)13-7-8-14-24-17-11-20(12-18-24)22(4,5)6/h9-12,15-18H,7-8,13-14H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334310

(1,4-bis(4-tert.butylpyridinium)-but-(2Z)-ene-1,4-d...)Show SMILES CC(C)(C)c1cc[n+](C\C=C/C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H32N2/c1-21(2,3)19-9-15-23(16-10-19)13-7-8-14-24-17-11-20(12-18-24)22(4,5)6/h7-12,15-18H,13-14H2,1-6H3/q+2/b8-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334314

(1,5-bis(4-tert.butylpyridinium)-pent-1,5-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C23H36N2/c1-22(2,3)20-10-16-24(17-11-20)14-8-7-9-15-25-18-12-21(13-19-25)23(4,5)6/h10-13,16-19H,7-9,14-15H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334313

(1,4-bis(4-tert.butylpyridinium)-but-1,4-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H34N2/c1-21(2,3)19-9-15-23(16-10-19)13-7-8-14-24-17-11-20(12-18-24)22(4,5)6/h9-12,15-18H,7-8,13-14H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334311

(1,1'-bis(4-tert.butylpyridinium)-1,2-phenyldimethy...)Show SMILES CC(C)(C)c1cc[n+](Cc2ccccc2C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H34N2/c1-25(2,3)23-11-15-27(16-12-23)19-21-9-7-8-10-22(21)20-28-17-13-24(14-18-28)26(4,5)6/h7-18H,19-20H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334315

(1,3-bis(4-tert.butylpyridinium)-2-oxaprop-1,3-diyl...)Show SMILES CC(C)(C)c1cc[n+](COC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C20H30N2O/c1-19(2,3)17-7-11-21(12-8-17)15-23-16-22-13-9-18(10-14-22)20(4,5)6/h7-14H,15-16H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334316

(1,3-bis(4-tert.butylpyridinium)-prop-1,3-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C21H32N2/c1-20(2,3)18-8-14-22(15-9-18)12-7-13-23-16-10-19(11-17-23)21(4,5)6/h8-11,14-17H,7,12-13H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334317

(1,2-bis(4-tert.butylpyridinium)-eth-1,2-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C20H30N2/c1-19(2,3)17-7-11-21(12-8-17)15-16-22-13-9-18(10-14-22)20(4,5)6/h7-14H,15-16H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334312

(1,4-bis(4-tert.butylpyridinium)-but-(2E)-ene-1,4-d...)Show SMILES CC(C)(C)c1cc[n+](C\C=C\C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H32N2/c1-21(2,3)19-9-15-23(16-10-19)13-7-8-14-24-17-11-20(12-18-24)22(4,5)6/h7-12,15-18H,13-14H2,1-6H3/q+2/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334318
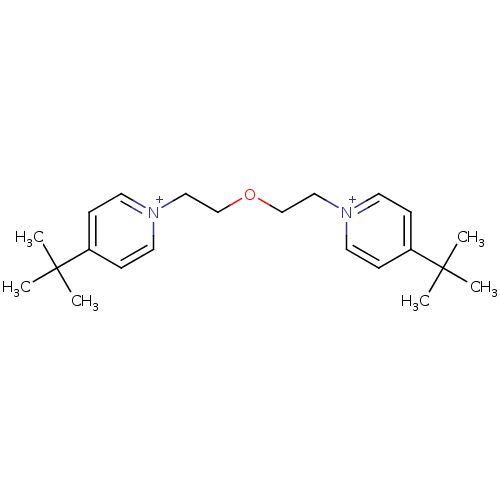
(1,5-bis(4-tert.butylpyridinium)-3-oxapent-1,5-diyl...)Show SMILES CC(C)(C)c1cc[n+](CCOCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H34N2O/c1-21(2,3)19-7-11-23(12-8-19)15-17-25-18-16-24-13-9-20(10-14-24)22(4,5)6/h7-14H,15-18H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334319
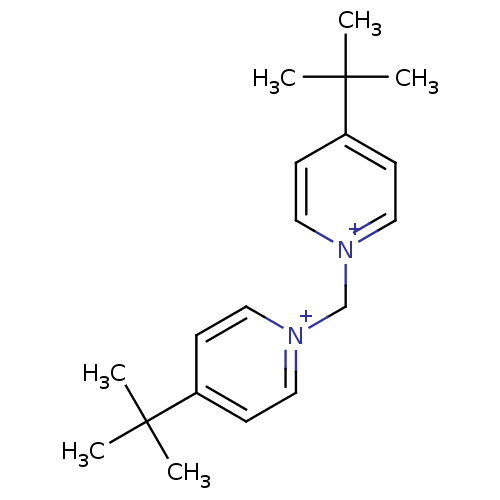
(1,1-bis(4-tert.butylpyridinium)-meth-1,1-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C19H28N2/c1-18(2,3)16-7-11-20(12-8-16)15-21-13-9-17(10-14-21)19(4,5)6/h7-14H,15H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334317

(1,2-bis(4-tert.butylpyridinium)-eth-1,2-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C20H30N2/c1-19(2,3)17-7-11-21(12-8-17)15-16-22-13-9-18(10-14-22)20(4,5)6/h7-14H,15-16H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334314

(1,5-bis(4-tert.butylpyridinium)-pent-1,5-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C23H36N2/c1-22(2,3)20-10-16-24(17-11-20)14-8-7-9-15-25-18-12-21(13-19-25)23(4,5)6/h10-13,16-19H,7-9,14-15H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334319

(1,1-bis(4-tert.butylpyridinium)-meth-1,1-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](C[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C19H28N2/c1-18(2,3)16-7-11-20(12-8-16)15-21-13-9-17(10-14-21)19(4,5)6/h7-14H,15H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334316

(1,3-bis(4-tert.butylpyridinium)-prop-1,3-diyl dibr...)Show SMILES CC(C)(C)c1cc[n+](CCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C21H32N2/c1-20(2,3)18-8-14-22(15-9-18)12-7-13-23-16-10-19(11-17-23)21(4,5)6/h8-11,14-17H,7,12-13H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334315

(1,3-bis(4-tert.butylpyridinium)-2-oxaprop-1,3-diyl...)Show SMILES CC(C)(C)c1cc[n+](COC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C20H30N2O/c1-19(2,3)17-7-11-21(12-8-17)15-23-16-22-13-9-18(10-14-22)20(4,5)6/h7-14H,15-16H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334318

(1,5-bis(4-tert.butylpyridinium)-3-oxapent-1,5-diyl...)Show SMILES CC(C)(C)c1cc[n+](CCOCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H34N2O/c1-21(2,3)19-7-11-23(12-8-19)15-17-25-18-16-24-13-9-20(10-14-24)22(4,5)6/h7-14H,15-18H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data