 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50039467
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50039467 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350022
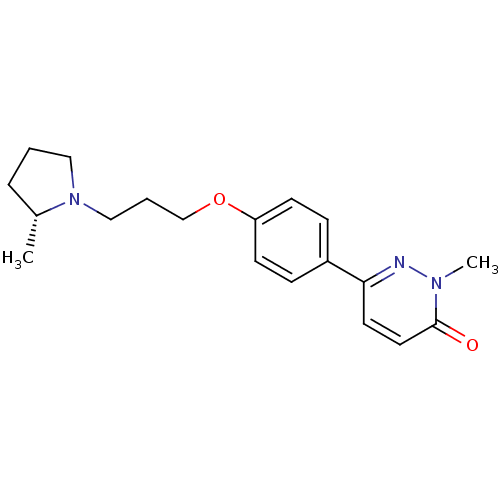
(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50262939
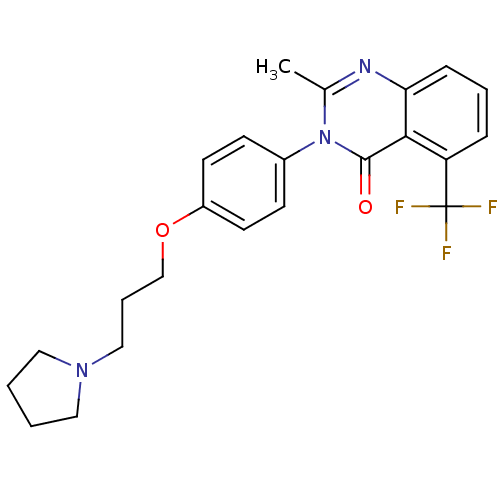
(2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...)Show SMILES Cc1nc2cccc(c2c(=O)n1-c1ccc(OCCCN2CCCC2)cc1)C(F)(F)F Show InChI InChI=1S/C23H24F3N3O2/c1-16-27-20-7-4-6-19(23(24,25)26)21(20)22(30)29(16)17-8-10-18(11-9-17)31-15-5-14-28-12-2-3-13-28/h4,6-11H,2-3,5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human H3 receptor |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350021
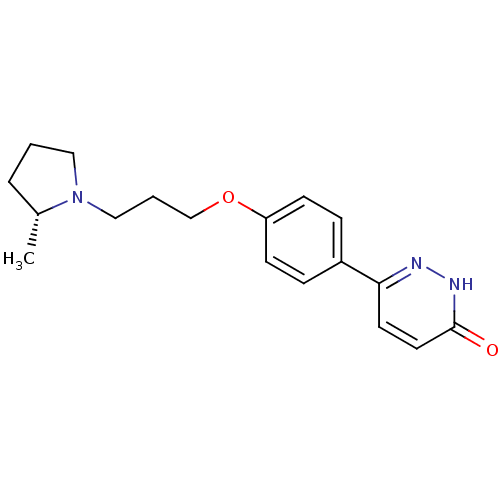
(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350030
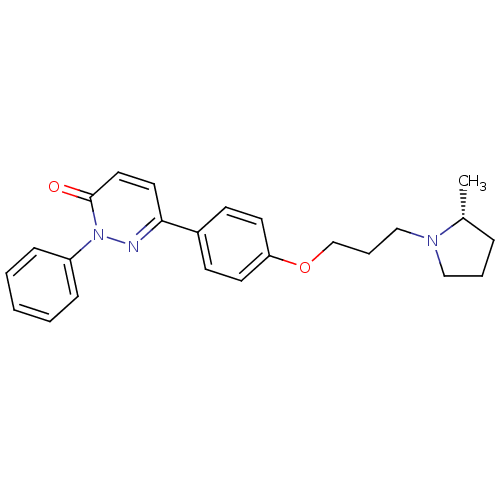
(CHEMBL1813060)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccccc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-19-7-5-16-26(19)17-6-18-29-22-12-10-20(11-13-22)23-14-15-24(28)27(25-23)21-8-3-2-4-9-21/h2-4,8-15,19H,5-7,16-18H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H3 receptor in rat cortical membrane |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350023
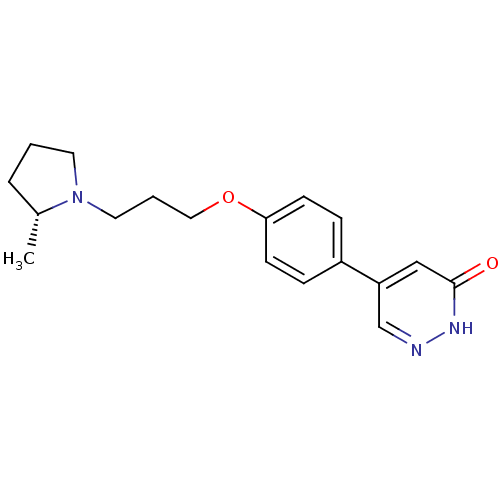
(CHEMBL1813065)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cn[nH]c(=O)c1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-9-21(14)10-3-11-23-17-7-5-15(6-8-17)16-12-18(22)20-19-13-16/h5-8,12-14H,2-4,9-11H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350028

(CHEMBL1813058)Show SMILES CCn1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C20H27N3O2/c1-3-23-20(24)12-11-19(21-23)17-7-9-18(10-8-17)25-15-5-14-22-13-4-6-16(22)2/h7-12,16H,3-6,13-15H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350029

(CHEMBL1813059)Show SMILES CC(C)n1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C21H29N3O2/c1-16(2)24-21(25)12-11-20(22-24)18-7-9-19(10-8-18)26-15-5-14-23-13-4-6-17(23)3/h7-12,16-17H,4-6,13-15H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350022

(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350031
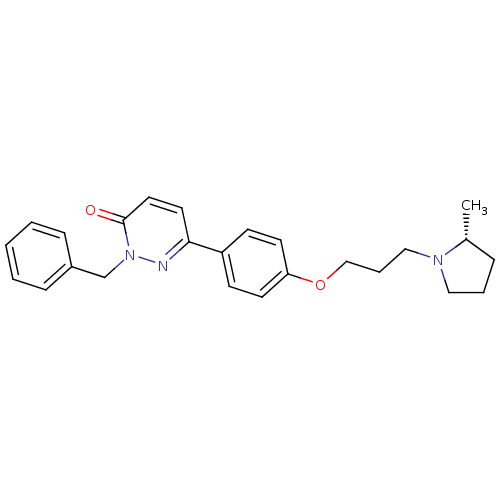
(CHEMBL1813061)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(Cc2ccccc2)n1 |r| Show InChI InChI=1S/C25H29N3O2/c1-20-7-5-16-27(20)17-6-18-30-23-12-10-22(11-13-23)24-14-15-25(29)28(26-24)19-21-8-3-2-4-9-21/h2-4,8-15,20H,5-7,16-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350029

(CHEMBL1813059)Show SMILES CC(C)n1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C21H29N3O2/c1-16(2)24-21(25)12-11-20(22-24)18-7-9-19(10-8-18)26-15-5-14-23-13-4-6-17(23)3/h7-12,16-17H,4-6,13-15H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350030

(CHEMBL1813060)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccccc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-19-7-5-16-26(19)17-6-18-29-22-12-10-20(11-13-22)23-14-15-24(28)27(25-23)21-8-3-2-4-9-21/h2-4,8-15,19H,5-7,16-18H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350023

(CHEMBL1813065)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cn[nH]c(=O)c1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-9-21(14)10-3-11-23-17-7-5-15(6-8-17)16-12-18(22)20-19-13-16/h5-8,12-14H,2-4,9-11H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350026

(CHEMBL1813055)Show InChI InChI=1S/C18H23N3O2/c22-18-10-9-17(19-20-18)15-5-7-16(8-6-15)23-14-4-13-21-11-2-1-3-12-21/h5-10H,1-4,11-14H2,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350033
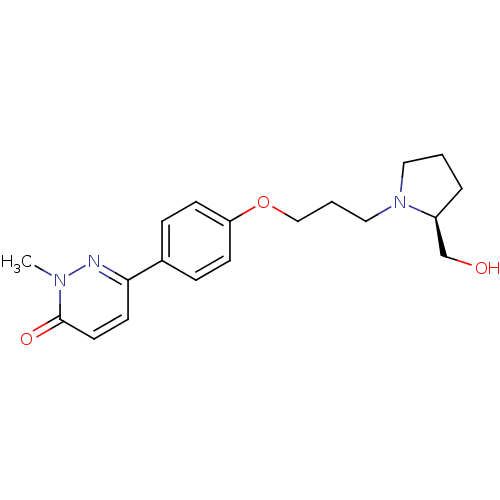
(CHEMBL1813063)Show SMILES Cn1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@H]2CO)cc1 |r| Show InChI InChI=1S/C19H25N3O3/c1-21-19(24)10-9-18(20-21)15-5-7-17(8-6-15)25-13-3-12-22-11-2-4-16(22)14-23/h5-10,16,23H,2-4,11-14H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350028

(CHEMBL1813058)Show SMILES CCn1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C20H27N3O2/c1-3-23-20(24)12-11-19(21-23)17-7-9-18(10-8-17)25-15-5-14-22-13-4-6-16(22)2/h7-12,16H,3-6,13-15H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350031

(CHEMBL1813061)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(Cc2ccccc2)n1 |r| Show InChI InChI=1S/C25H29N3O2/c1-20-7-5-16-27(20)17-6-18-30-23-12-10-22(11-13-23)24-14-15-25(29)28(26-24)19-21-8-3-2-4-9-21/h2-4,8-15,20H,5-7,16-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350024

(CHEMBL1813053)Show SMILES C[C@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350025

(CHEMBL1813054)Show InChI InChI=1S/C17H21N3O2/c21-17-9-8-16(18-19-17)14-4-6-15(7-5-14)22-13-3-12-20-10-1-2-11-20/h4-9H,1-3,10-13H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350032

(CHEMBL1813062)Show InChI InChI=1S/C19H25N3O2/c1-21-19(23)11-10-18(20-21)16-6-8-17(9-7-16)24-15-5-14-22-12-3-2-4-13-22/h6-11H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350026

(CHEMBL1813055)Show InChI InChI=1S/C18H23N3O2/c22-18-10-9-17(19-20-18)15-5-7-16(8-6-15)23-14-4-13-21-11-2-1-3-12-21/h5-10H,1-4,11-14H2,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350025

(CHEMBL1813054)Show InChI InChI=1S/C17H21N3O2/c21-17-9-8-16(18-19-17)14-4-6-15(7-5-14)22-13-3-12-20-10-1-2-11-20/h4-9H,1-3,10-13H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350033

(CHEMBL1813063)Show SMILES Cn1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@H]2CO)cc1 |r| Show InChI InChI=1S/C19H25N3O3/c1-21-19(24)10-9-18(20-21)15-5-7-17(8-6-15)25-13-3-12-22-11-2-4-16(22)14-23/h5-10,16,23H,2-4,11-14H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350024

(CHEMBL1813053)Show SMILES C[C@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350035

(CHEMBL1813066)Show InChI InChI=1S/C18H23N3O2/c1-15-5-3-12-20(15)13-4-14-23-17-9-7-16(8-10-17)21-18(22)6-2-11-19-21/h2,6-11,15H,3-5,12-14H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350032

(CHEMBL1813062)Show InChI InChI=1S/C19H25N3O2/c1-21-19(23)11-10-18(20-21)16-6-8-17(9-7-16)24-15-5-14-22-12-3-2-4-13-22/h6-11H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350034

(CHEMBL1813064)Show SMILES Cn1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@@H]2CO)cc1 |r| Show InChI InChI=1S/C19H25N3O3/c1-21-19(24)10-9-18(20-21)15-5-7-17(8-6-15)25-13-3-12-22-11-2-4-16(22)14-23/h5-10,16,23H,2-4,11-14H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350035

(CHEMBL1813066)Show InChI InChI=1S/C18H23N3O2/c1-15-5-3-12-20(15)13-4-14-23-17-9-7-16(8-10-17)21-18(22)6-2-11-19-21/h2,6-11,15H,3-5,12-14H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350034

(CHEMBL1813064)Show SMILES Cn1nc(ccc1=O)-c1ccc(OCCCN2CCC[C@@H]2CO)cc1 |r| Show InChI InChI=1S/C19H25N3O3/c1-21-19(24)10-9-18(20-21)15-5-7-17(8-6-15)25-13-3-12-22-11-2-4-16(22)14-23/h5-10,16,23H,2-4,11-14H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350027

(CHEMBL1813056)Show InChI InChI=1S/C17H21N3O3/c21-17-7-6-16(18-19-17)14-2-4-15(5-3-14)23-11-1-8-20-9-12-22-13-10-20/h2-7H,1,8-13H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 767 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350027

(CHEMBL1813056)Show InChI InChI=1S/C17H21N3O3/c21-17-7-6-16(18-19-17)14-2-4-15(5-3-14)23-11-1-8-20-9-12-22-13-10-20/h2-7H,1,8-13H2,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 822 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic M2 receptor |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of adrenergic alpha 1A receptor |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine transporter |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine transporter |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human dopamine transporter |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350023

(CHEMBL1813065)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cn[nH]c(=O)c1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-9-21(14)10-3-11-23-17-7-5-15(6-8-17)16-12-18(22)20-19-13-16/h5-8,12-14H,2-4,9-11H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ERG channel expressed in human HEK 293 cells by patch clamp assay |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350022

(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ERG channel expressed in human HEK 293 cells by patch clamp assay |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ERG channel expressed in human HEK 293 cells by patch clamp assay |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human norepinephrine transporter |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50350021

(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350022

(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine induced [35S]GTPgammaS binding |
J Med Chem 54: 4781-92 (2011)
Article DOI: 10.1021/jm200401v
BindingDB Entry DOI: 10.7270/Q2W66MRB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data