 Found 66 hits Enz. Inhib. hit(s) with all data for entry = 50039789
Found 66 hits Enz. Inhib. hit(s) with all data for entry = 50039789 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384144
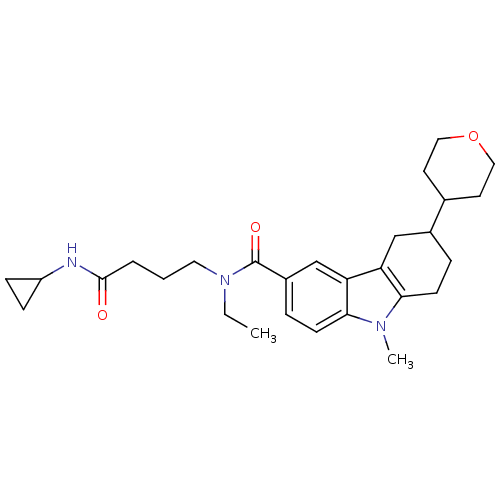
(CHEMBL2029719)Show SMILES CCN(CCCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C28H39N3O3/c1-3-31(14-4-5-27(32)29-22-8-9-22)28(33)21-7-11-26-24(18-21)23-17-20(6-10-25(23)30(26)2)19-12-15-34-16-13-19/h7,11,18-20,22H,3-6,8-10,12-17H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384161
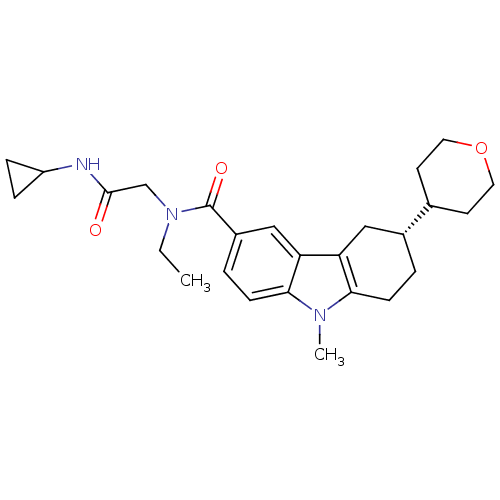
(CHEMBL2029727)Show SMILES CCN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H35N3O3/c1-3-29(16-25(30)27-20-6-7-20)26(31)19-5-9-24-22(15-19)21-14-18(4-8-23(21)28(24)2)17-10-12-32-13-11-17/h5,9,15,17-18,20H,3-4,6-8,10-14,16H2,1-2H3,(H,27,30)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384143

(CHEMBL2029718)Show SMILES CN(CCCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C27H37N3O3/c1-29(13-3-4-26(31)28-21-7-8-21)27(32)20-6-10-25-23(17-20)22-16-19(5-9-24(22)30(25)2)18-11-14-33-15-12-18/h6,10,17-19,21H,3-5,7-9,11-16H2,1-2H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384146
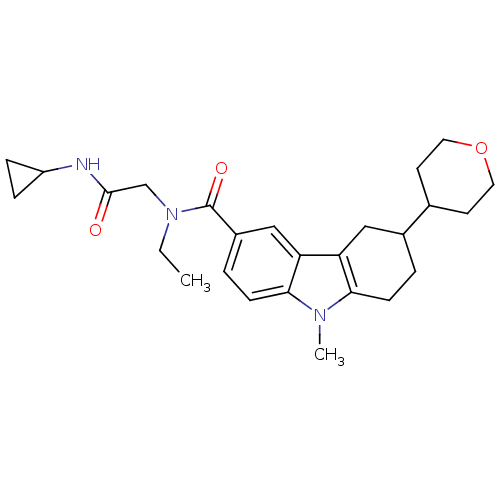
(CHEMBL2029722)Show SMILES CCN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O3/c1-3-29(16-25(30)27-20-6-7-20)26(31)19-5-9-24-22(15-19)21-14-18(4-8-23(21)28(24)2)17-10-12-32-13-11-17/h5,9,15,17-18,20H,3-4,6-8,10-14,16H2,1-2H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364920
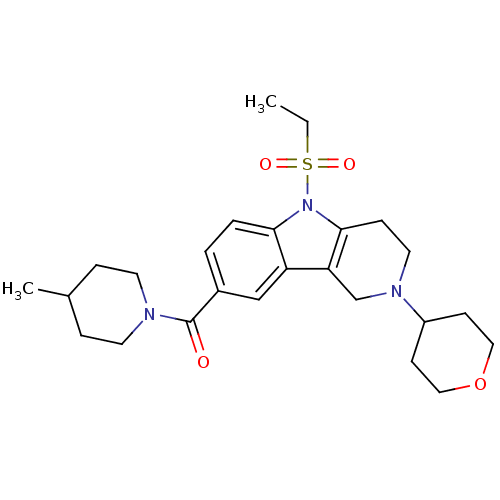
(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50384159

(CHEMBL2029729)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384160
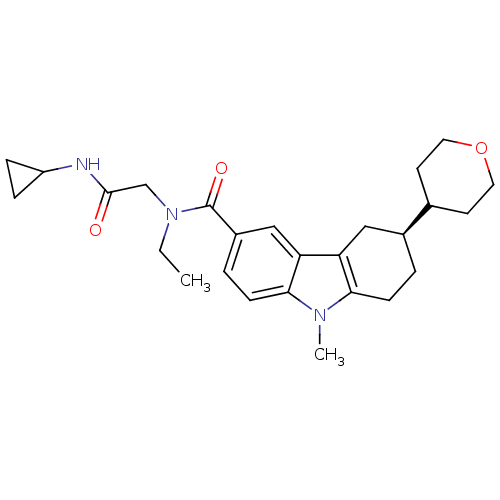
(CHEMBL2029728)Show SMILES CCN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H35N3O3/c1-3-29(16-25(30)27-20-6-7-20)26(31)19-5-9-24-22(15-19)21-14-18(4-8-23(21)28(24)2)17-10-12-32-13-11-17/h5,9,15,17-18,20H,3-4,6-8,10-14,16H2,1-2H3,(H,27,30)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50218116
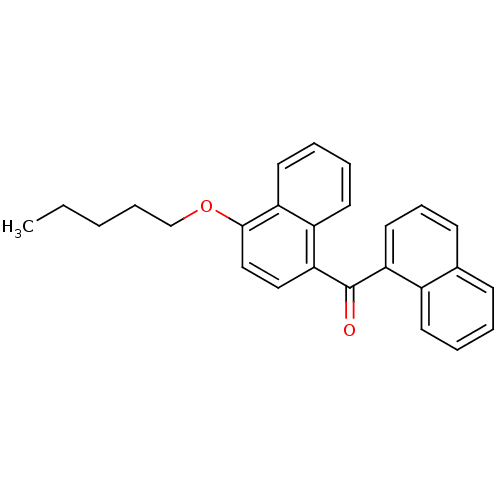
(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50384149
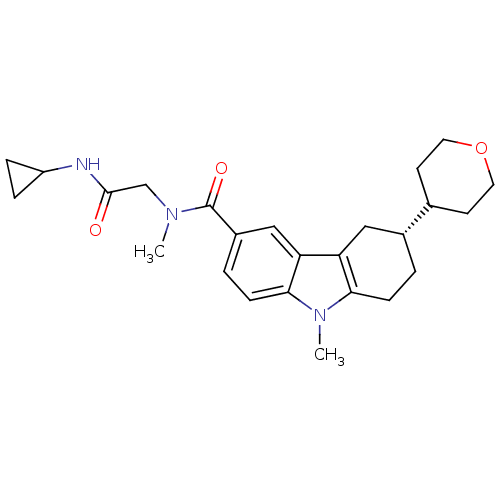
(CHEMBL2029725)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384149

(CHEMBL2029725)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50384149

(CHEMBL2029725)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384159

(CHEMBL2029729)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50218116

(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384149

(CHEMBL2029725)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50384150
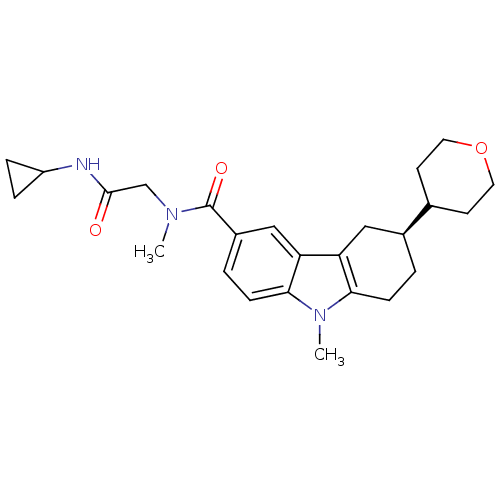
(CHEMBL2029726)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384148
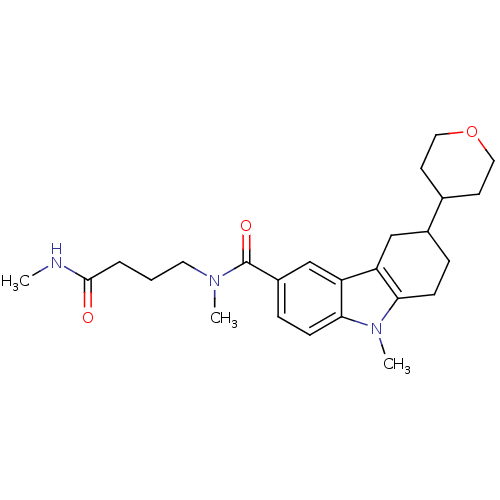
(CHEMBL2029724)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384150

(CHEMBL2029726)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384162
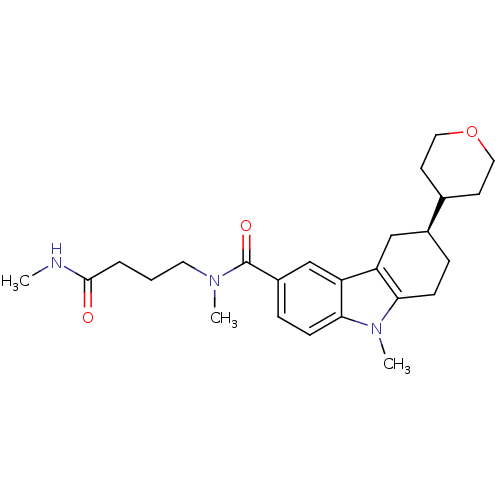
(CHEMBL2029730)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 576 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384147
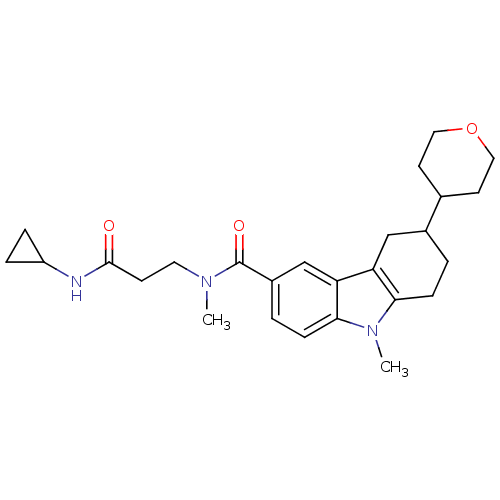
(CHEMBL2029723)Show SMILES CN(CCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O3/c1-28(12-9-25(30)27-20-5-6-20)26(31)19-4-8-24-22(16-19)21-15-18(3-7-23(21)29(24)2)17-10-13-32-14-11-17/h4,8,16-18,20H,3,5-7,9-15H2,1-2H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 655 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384145
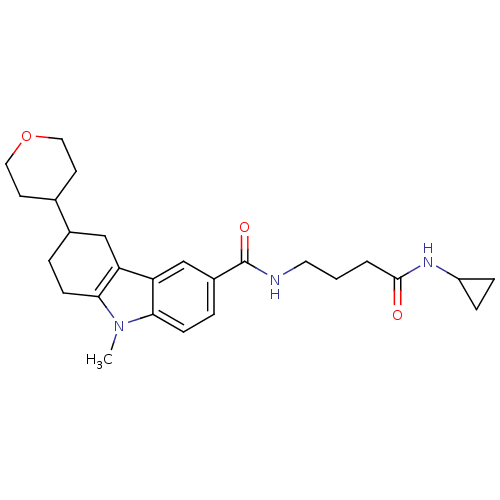
(CHEMBL2029720)Show SMILES Cn1c2CCC(Cc2c2cc(ccc12)C(=O)NCCCC(=O)NC1CC1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O3/c1-29-23-8-4-18(17-10-13-32-14-11-17)15-21(23)22-16-19(5-9-24(22)29)26(31)27-12-2-3-25(30)28-20-6-7-20/h5,9,16-18,20H,2-4,6-8,10-15H2,1H3,(H,27,31)(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50384159

(CHEMBL2029729)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384145

(CHEMBL2029720)Show SMILES Cn1c2CCC(Cc2c2cc(ccc12)C(=O)NCCCC(=O)NC1CC1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O3/c1-29-23-8-4-18(17-10-13-32-14-11-17)15-21(23)22-16-19(5-9-24(22)29)26(31)27-12-2-3-25(30)28-20-6-7-20/h5,9,16-18,20H,2-4,6-8,10-15H2,1H3,(H,27,31)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384152
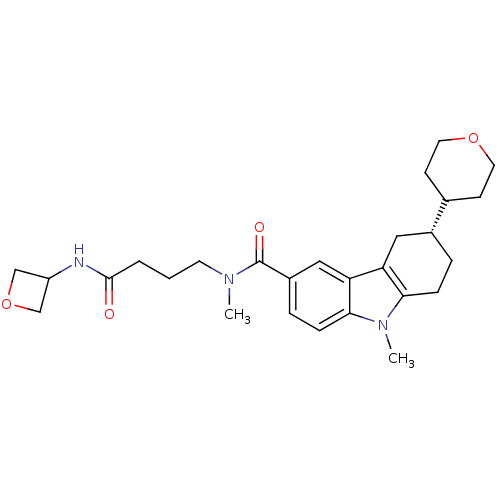
(CHEMBL2029734)Show SMILES CN(CCCC(=O)NC1COC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H37N3O4/c1-29(11-3-4-26(31)28-21-16-34-17-21)27(32)20-6-8-25-23(15-20)22-14-19(5-7-24(22)30(25)2)18-9-12-33-13-10-18/h6,8,15,18-19,21H,3-5,7,9-14,16-17H2,1-2H3,(H,28,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384151

(CHEMBL2029733)Show SMILES CCN(CCCC(=O)NCCO)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H39N3O4/c1-3-30(13-4-5-26(32)28-12-14-31)27(33)21-7-9-25-23(18-21)22-17-20(6-8-24(22)29(25)2)19-10-15-34-16-11-19/h7,9,18-20,31H,3-6,8,10-17H2,1-2H3,(H,28,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384150

(CHEMBL2029726)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384149

(CHEMBL2029725)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384148

(CHEMBL2029724)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384146

(CHEMBL2029722)Show SMILES CCN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O3/c1-3-29(16-25(30)27-20-6-7-20)26(31)19-5-9-24-22(15-19)21-14-18(4-8-23(21)28(24)2)17-10-12-32-13-11-17/h5,9,15,17-18,20H,3-4,6-8,10-14,16H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384153

(CHEMBL2029735)Show SMILES CN([C@H](CO)CCC(=O)NCCF)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H38FN3O4/c1-30(21(17-32)5-8-26(33)29-12-11-28)27(34)20-4-7-25-23(16-20)22-15-19(3-6-24(22)31(25)2)18-9-13-35-14-10-18/h4,7,16,18-19,21,32H,3,5-6,8-15,17H2,1-2H3,(H,29,33)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384154
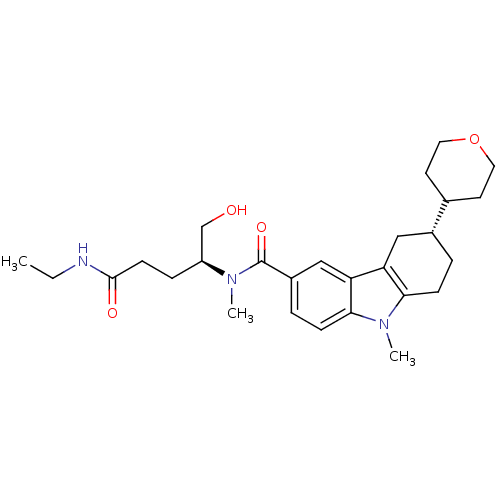
(CHEMBL2029736)Show SMILES CCNC(=O)CC[C@@H](CO)N(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H39N3O4/c1-4-28-26(32)10-7-21(17-31)29(2)27(33)20-6-9-25-23(16-20)22-15-19(5-8-24(22)30(25)3)18-11-13-34-14-12-18/h6,9,16,18-19,21,31H,4-5,7-8,10-15,17H2,1-3H3,(H,28,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384155

(CHEMBL2029737)Show SMILES CNC(=O)CC[C@@H](CO)N(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H37N3O4/c1-27-25(31)9-6-20(16-30)28(2)26(32)19-5-8-24-22(15-19)21-14-18(4-7-23(21)29(24)3)17-10-12-33-13-11-17/h5,8,15,17-18,20,30H,4,6-7,9-14,16H2,1-3H3,(H,27,31)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384156
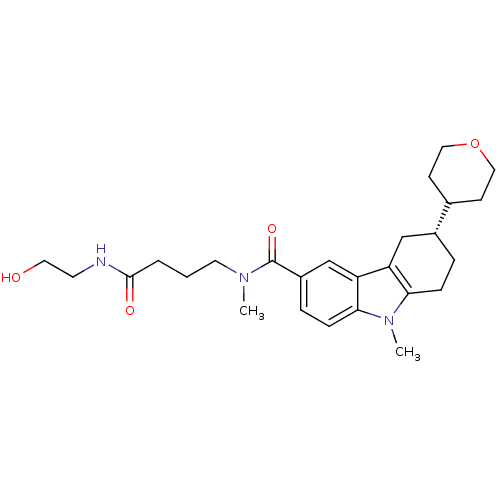
(CHEMBL2029738)Show SMILES CN(CCCC(=O)NCCO)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H37N3O4/c1-28(12-3-4-25(31)27-11-13-30)26(32)20-6-8-24-22(17-20)21-16-19(5-7-23(21)29(24)2)18-9-14-33-15-10-18/h6,8,17-19,30H,3-5,7,9-16H2,1-2H3,(H,27,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384157
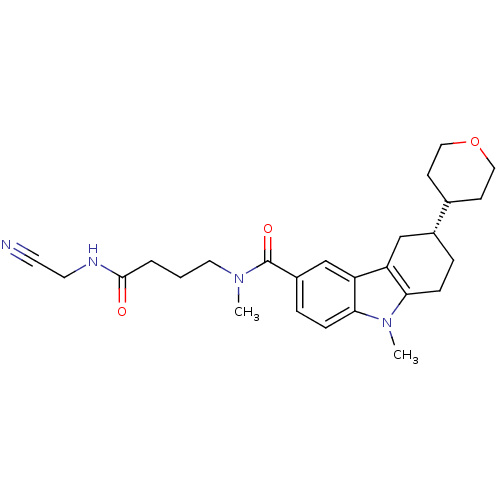
(CHEMBL2029739)Show SMILES CN(CCCC(=O)NCC#N)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H34N4O3/c1-29(13-3-4-25(31)28-12-11-27)26(32)20-6-8-24-22(17-20)21-16-19(5-7-23(21)30(24)2)18-9-14-33-15-10-18/h6,8,17-19H,3-5,7,9-10,12-16H2,1-2H3,(H,28,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384158

(CHEMBL2029899)Show SMILES CONC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O4/c1-27(12-4-5-24(29)26-31-3)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)2)17-10-13-32-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384149

(CHEMBL2029725)Show SMILES CN(CC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H33N3O3/c1-27(15-24(29)26-19-5-6-19)25(30)18-4-8-23-21(14-18)20-13-17(3-7-22(20)28(23)2)16-9-11-31-12-10-16/h4,8,14,16-17,19H,3,5-7,9-13,15H2,1-2H3,(H,26,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384147

(CHEMBL2029723)Show SMILES CN(CCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C26H35N3O3/c1-28(12-9-25(30)27-20-5-6-20)26(31)19-4-8-24-22(16-19)21-15-18(3-7-23(21)29(24)2)17-10-13-32-14-11-17/h4,8,16-18,20H,3,5-7,9-15H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384144

(CHEMBL2029719)Show SMILES CCN(CCCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C28H39N3O3/c1-3-31(14-4-5-27(32)29-22-8-9-22)28(33)21-7-11-26-24(18-21)23-17-20(6-10-25(23)30(26)2)19-12-15-34-16-13-19/h7,11,18-20,22H,3-6,8-10,12-17H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384143

(CHEMBL2029718)Show SMILES CN(CCCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C27H37N3O3/c1-29(13-3-4-26(31)28-21-7-8-21)27(32)20-6-10-25-23(17-20)22-16-19(5-9-24(22)30(25)2)18-11-14-33-15-12-18/h6,10,17-19,21H,3-5,7-9,11-16H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50384159

(CHEMBL2029729)Show SMILES CNC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O3/c1-26-24(29)5-4-12-27(2)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)3)17-10-13-31-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384158

(CHEMBL2029899)Show SMILES CONC(=O)CCCN(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O4/c1-27(12-4-5-24(29)26-31-3)25(30)19-7-9-23-21(16-19)20-15-18(6-8-22(20)28(23)2)17-10-13-32-14-11-17/h7,9,16-18H,4-6,8,10-15H2,1-3H3,(H,26,29)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 291 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384157

(CHEMBL2029739)Show SMILES CN(CCCC(=O)NCC#N)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H34N4O3/c1-29(13-3-4-25(31)28-12-11-27)26(32)20-6-8-24-22(17-20)21-16-19(5-7-23(21)30(24)2)18-9-14-33-15-10-18/h6,8,17-19H,3-5,7,9-10,12-16H2,1-2H3,(H,28,31)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70.6 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384156

(CHEMBL2029738)Show SMILES CN(CCCC(=O)NCCO)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H37N3O4/c1-28(12-3-4-25(31)27-11-13-30)26(32)20-6-8-24-22(17-20)21-16-19(5-7-23(21)29(24)2)18-9-14-33-15-10-18/h6,8,17-19,30H,3-5,7,9-16H2,1-2H3,(H,27,31)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 173 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384155

(CHEMBL2029737)Show SMILES CNC(=O)CC[C@@H](CO)N(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C26H37N3O4/c1-27-25(31)9-6-20(16-30)28(2)26(32)19-5-8-24-22(15-19)21-14-18(4-7-23(21)29(24)3)17-10-12-33-13-11-17/h5,8,15,17-18,20,30H,4,6-7,9-14,16H2,1-3H3,(H,27,31)/t18-,20+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 101 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384154

(CHEMBL2029736)Show SMILES CCNC(=O)CC[C@@H](CO)N(C)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H39N3O4/c1-4-28-26(32)10-7-21(17-31)29(2)27(33)20-6-9-25-23(16-20)22-15-19(5-8-24(22)30(25)3)18-11-13-34-14-12-18/h6,9,16,18-19,21,31H,4-5,7-8,10-15,17H2,1-3H3,(H,28,32)/t19-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 78.7 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384153

(CHEMBL2029735)Show SMILES CN([C@H](CO)CCC(=O)NCCF)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H38FN3O4/c1-30(21(17-32)5-8-26(33)29-12-11-28)27(34)20-4-7-25-23(16-20)22-15-19(3-6-24(22)31(25)2)18-9-13-35-14-10-18/h4,7,16,18-19,21,32H,3,5-6,8-15,17H2,1-2H3,(H,29,33)/t19-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 61.4 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50384152

(CHEMBL2029734)Show SMILES CN(CCCC(=O)NC1COC1)C(=O)c1ccc2n(C)c3CC[C@H](Cc3c2c1)C1CCOCC1 |r| Show InChI InChI=1S/C27H37N3O4/c1-29(11-3-4-26(31)28-21-16-34-17-21)27(32)20-6-8-25-23(15-20)22-14-19(5-7-24(22)30(25)2)18-9-12-33-13-10-18/h6,8,15,18-19,21H,3-5,7,9-14,16-17H2,1-2H3,(H,28,31)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as [35S]GTPgamma binding |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data