 Found 16 hits Enz. Inhib. hit(s) with all data for entry = 50039824
Found 16 hits Enz. Inhib. hit(s) with all data for entry = 50039824 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163460

(2-Amino-6-(2-hydroxy-ethylsulfanyl)-4-(3-trifluoro...)Show SMILES Nc1nc(SCCO)c(C#N)c(-c2cccc(c2)C(F)(F)F)c1C#N Show InChI InChI=1S/C16H11F3N4OS/c17-16(18,19)10-3-1-2-9(6-10)13-11(7-20)14(22)23-15(12(13)8-21)25-5-4-24/h1-3,6,24H,4-5H2,(H2,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163453
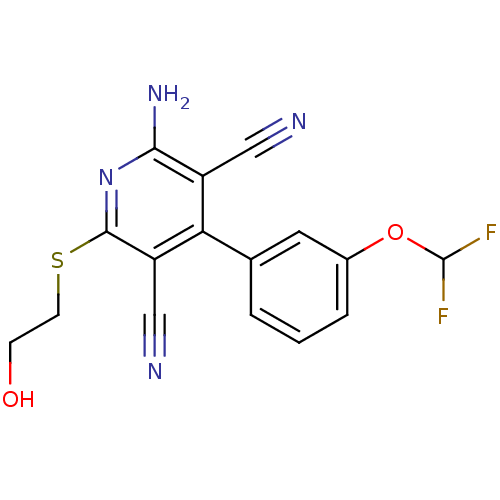
(2-Amino-4-(3-difluoromethoxy-phenyl)-6-(2-hydroxy-...)Show InChI InChI=1S/C16H12F2N4O2S/c17-16(18)24-10-3-1-2-9(6-10)13-11(7-19)14(21)22-15(12(13)8-20)25-5-4-23/h1-3,6,16,23H,4-5H2,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163452
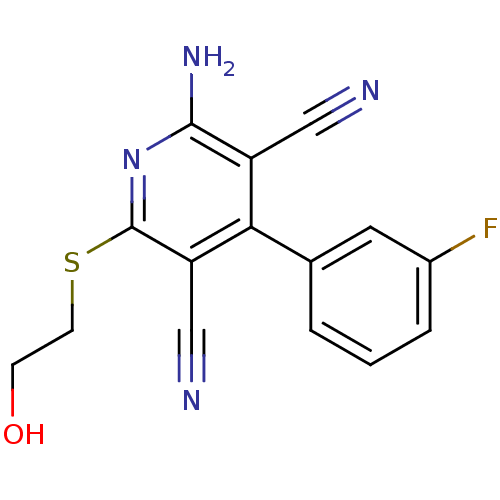
(2-Amino-4-(3-fluoro-phenyl)-6-(2-hydroxy-ethylsulf...)Show InChI InChI=1S/C15H11FN4OS/c16-10-3-1-2-9(6-10)13-11(7-17)14(19)20-15(12(13)8-18)22-5-4-21/h1-3,6,21H,4-5H2,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50163462
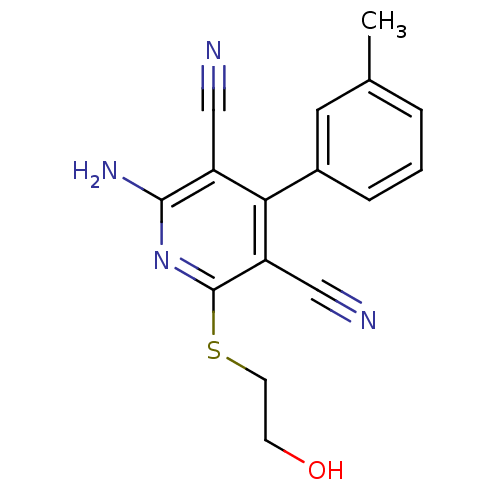
(2-Amino-6-(2-hydroxy-ethylsulfanyl)-4-m-tolyl-pyri...)Show InChI InChI=1S/C16H14N4OS/c1-10-3-2-4-11(7-10)14-12(8-17)15(19)20-16(13(14)9-18)22-6-5-21/h2-4,7,21H,5-6H2,1H3,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13066
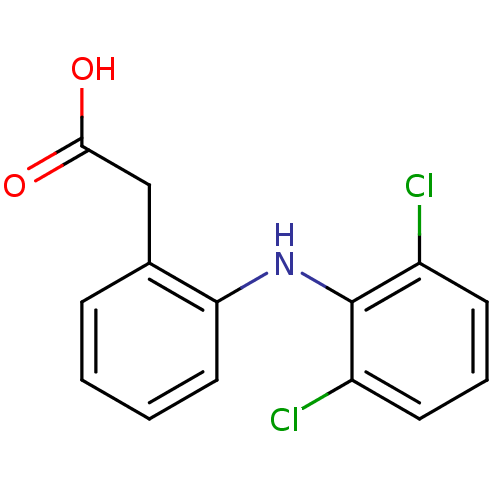
(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13066

(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM5380
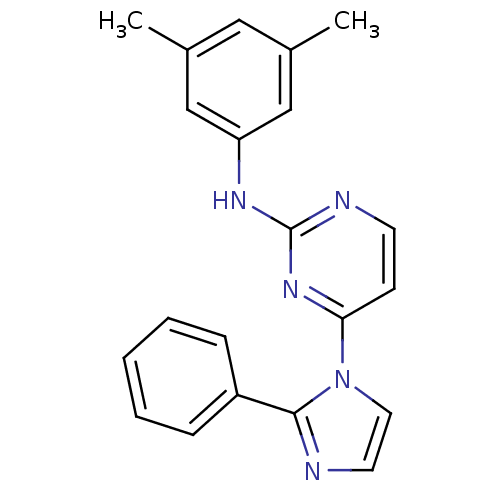
(2,4-Disubstituted Pyrimidine 2d | N-(3,5-dimethylp...)Show InChI InChI=1S/C21H19N5/c1-15-12-16(2)14-18(13-15)24-21-23-9-8-19(25-21)26-11-10-22-20(26)17-6-4-3-5-7-17/h3-14H,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at VEGFR2 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50207446
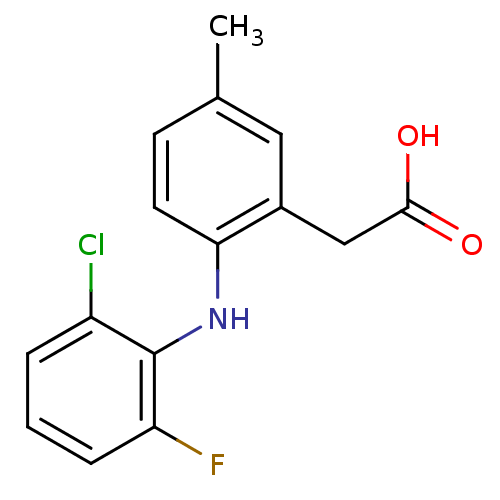
(2-(2-(2-chloro-6-fluorophenylamino)-5-methylphenyl...)Show InChI InChI=1S/C15H13ClFNO2/c1-9-5-6-13(10(7-9)8-14(19)20)18-15-11(16)3-2-4-12(15)17/h2-7,18H,8H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50384799
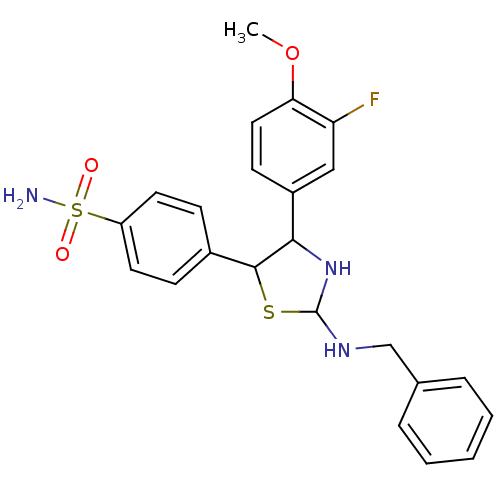
(CHEMBL2037483)Show SMILES COc1ccc(cc1F)C1NC(NCc2ccccc2)SC1c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C23H24FN3O3S2/c1-30-20-12-9-17(13-19(20)24)21-22(16-7-10-18(11-8-16)32(25,28)29)31-23(27-21)26-14-15-5-3-2-4-6-15/h2-13,21-23,26-27H,14H2,1H3,(H2,25,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM5400
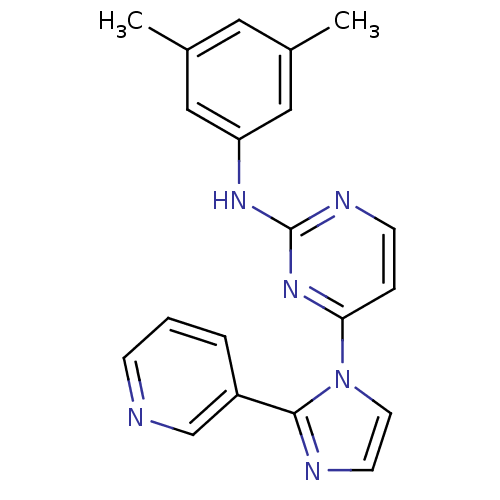
(2,4-Disubstituted Pyrimidine 5b | N-(3,5-dimethylp...)Show InChI InChI=1S/C20H18N6/c1-14-10-15(2)12-17(11-14)24-20-23-7-5-18(25-20)26-9-8-22-19(26)16-4-3-6-21-13-16/h3-13H,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Antagonist activity at VEGFR2 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19828
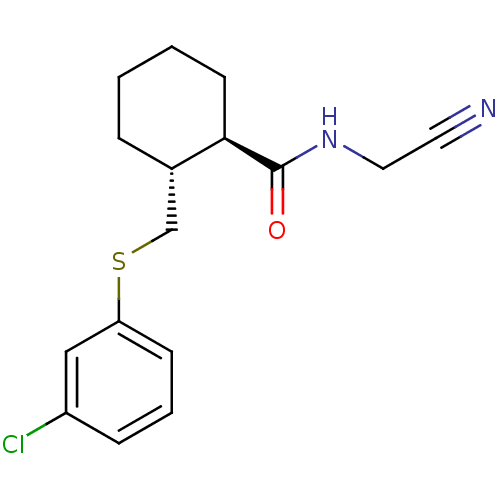
((1R,2R)-2-{[(3-chlorophenyl)sulfanyl]methyl}-N-(cy...)Show SMILES Clc1cccc(SC[C@@H]2CCCC[C@H]2C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C16H19ClN2OS/c17-13-5-3-6-14(10-13)21-11-12-4-1-2-7-15(12)16(20)19-9-8-18/h3,5-6,10,12,15H,1-2,4,7,9,11H2,(H,19,20)/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19817

((1R,2R)-N-(cyanomethyl)-2-[(phenylsulfanyl)methyl]...)Show InChI InChI=1S/C16H20N2OS/c17-10-11-18-16(19)15-9-5-4-6-13(15)12-20-14-7-2-1-3-8-14/h1-3,7-8,13,15H,4-6,9,11-12H2,(H,18,19)/t13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50207446

(2-(2-(2-chloro-6-fluorophenylamino)-5-methylphenyl...)Show InChI InChI=1S/C15H13ClFNO2/c1-9-5-6-13(10(7-9)8-14(19)20)18-15-11(16)3-2-4-12(15)17/h2-7,18H,8H2,1H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM19817

((1R,2R)-N-(cyanomethyl)-2-[(phenylsulfanyl)methyl]...)Show InChI InChI=1S/C16H20N2OS/c17-10-11-18-16(19)15-9-5-4-6-13(15)12-20-14-7-2-1-3-8-14/h1-3,7-8,13,15H,4-6,9,11-12H2,(H,18,19)/t13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049661
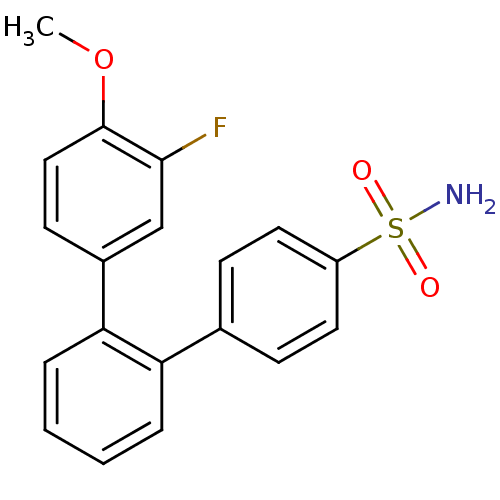
(3''''-Fluoro-4''''-methoxy-[1,1'';2'',1'''']terphe...)Show SMILES COc1ccc(cc1F)-c1ccccc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16FNO3S/c1-24-19-11-8-14(12-18(19)20)17-5-3-2-4-16(17)13-6-9-15(10-7-13)25(21,22)23/h2-12H,1H3,(H2,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19828

((1R,2R)-2-{[(3-chlorophenyl)sulfanyl]methyl}-N-(cy...)Show SMILES Clc1cccc(SC[C@@H]2CCCC[C@H]2C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C16H19ClN2OS/c17-13-5-3-6-14(10-13)21-11-12-4-1-2-7-15(12)16(20)19-9-8-18/h3,5-6,10,12,15H,1-2,4,7,9,11H2,(H,19,20)/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
J Med Chem 55: 2932-42 (2012)
Article DOI: 10.1021/jm201706b
BindingDB Entry DOI: 10.7270/Q2RJ4KGS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data