 Found 32 hits Enz. Inhib. hit(s) with all data for entry = 50040072
Found 32 hits Enz. Inhib. hit(s) with all data for entry = 50040072 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50388877
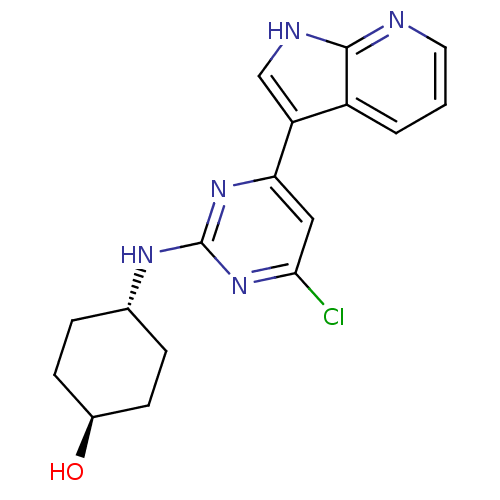
(CHEMBL2062936)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cl)cc(n1)-c1c[nH]c2ncccc12 |r,wU:4.7,wD:1.0,(29.96,-30.16,;29.85,-28.62,;29.57,-27.1,;27.75,-27.13,;26.77,-26.34,;27.05,-27.87,;28.79,-27.81,;25.98,-25.03,;26.73,-23.68,;28.27,-23.66,;29.01,-22.3,;30.55,-22.27,;28.22,-20.99,;26.69,-21.02,;25.94,-22.36,;25.9,-19.71,;24.37,-19.57,;24.02,-18.07,;25.34,-17.28,;25.64,-15.76,;27.1,-15.27,;28.26,-16.29,;27.96,-17.79,;26.5,-18.29,)| Show InChI InChI=1S/C17H18ClN5O/c18-15-8-14(13-9-20-16-12(13)2-1-7-19-16)22-17(23-15)21-10-3-5-11(24)6-4-10/h1-2,7-11,24H,3-6H2,(H,19,20)(H,21,22,23)/t10-,11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50388879
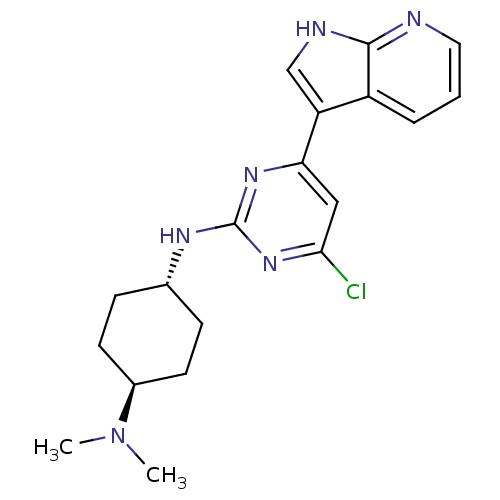
(CHEMBL2062937)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)Nc1nc(Cl)cc(n1)-c1c[nH]c2ncccc12 |r,wU:6.9,wD:3.2,(39.49,-29.31,;40.58,-28.23,;42.07,-28.64,;40.19,-26.75,;39.91,-25.24,;38.09,-25.26,;37.12,-24.48,;37.39,-26,;39.14,-25.95,;36.32,-23.16,;37.07,-21.81,;38.61,-21.79,;39.36,-20.43,;40.9,-20.4,;38.56,-19.12,;37.03,-19.15,;36.28,-20.5,;36.25,-17.83,;34.71,-17.7,;34.37,-16.2,;35.69,-15.41,;35.99,-13.89,;37.45,-13.39,;38.6,-14.42,;38.3,-15.92,;36.85,-16.42,)| Show InChI InChI=1S/C19H23ClN6/c1-26(2)13-7-5-12(6-8-13)23-19-24-16(10-17(20)25-19)15-11-22-18-14(15)4-3-9-21-18/h3-4,9-13H,5-8H2,1-2H3,(H,21,22)(H,23,24,25)/t12-,13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50388878
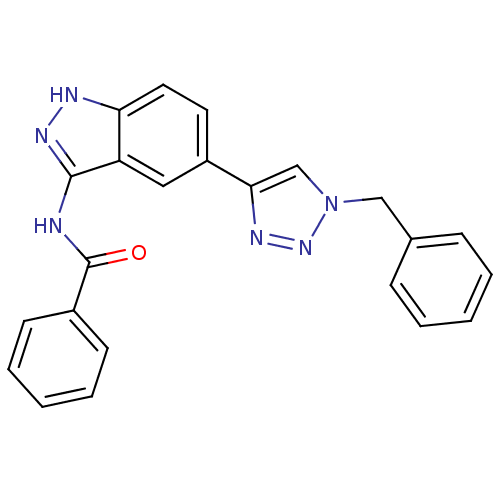
(CHEMBL1999931 | US9163007, 73)Show SMILES O=C(Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1)c1ccccc1 Show InChI InChI=1S/C23H18N6O/c30-23(17-9-5-2-6-10-17)24-22-19-13-18(11-12-20(19)25-27-22)21-15-29(28-26-21)14-16-7-3-1-4-8-16/h1-13,15H,14H2,(H2,24,25,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50388876
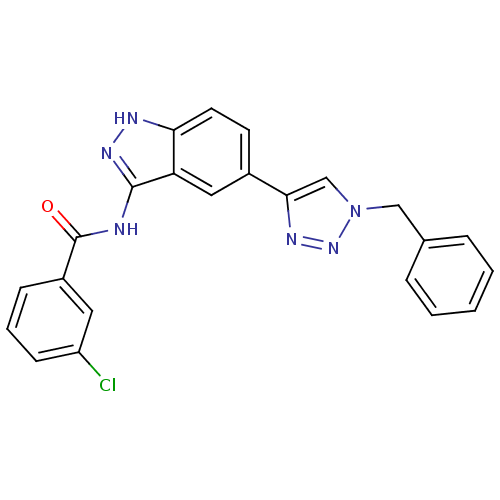
(CHEMBL1983595)Show SMILES Clc1cccc(c1)C(=O)Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1 Show InChI InChI=1S/C23H17ClN6O/c24-18-8-4-7-17(11-18)23(31)25-22-19-12-16(9-10-20(19)26-28-22)21-14-30(29-27-21)13-15-5-2-1-3-6-15/h1-12,14H,13H2,(H2,25,26,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50381719
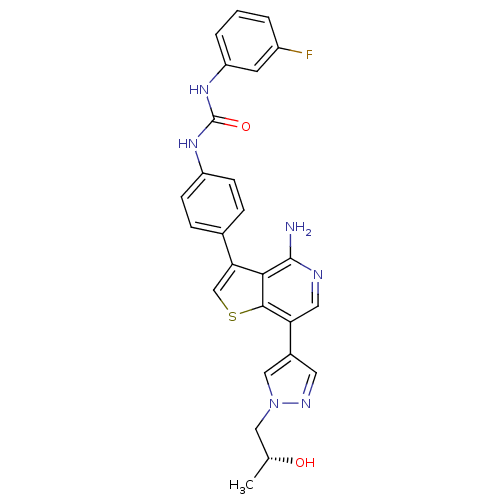
(CHEMBL2022856)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 |r| Show InChI InChI=1S/C26H23FN6O2S/c1-15(34)12-33-13-17(10-30-33)21-11-29-25(28)23-22(14-36-24(21)23)16-5-7-19(8-6-16)31-26(35)32-20-4-2-3-18(27)9-20/h2-11,13-15,34H,12H2,1H3,(H2,28,29)(H2,31,32,35)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50381716
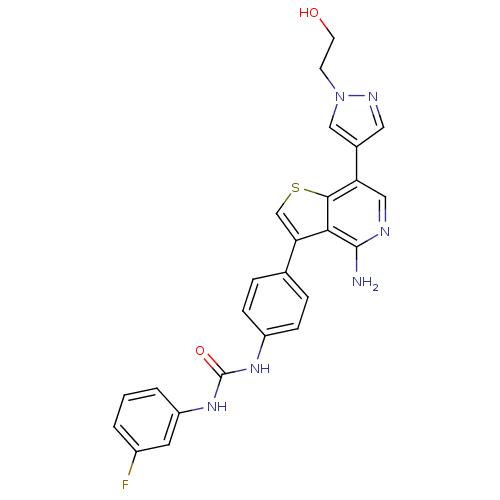
(ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...)Show SMILES Nc1ncc(-c2cnn(CCO)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRA |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50142887
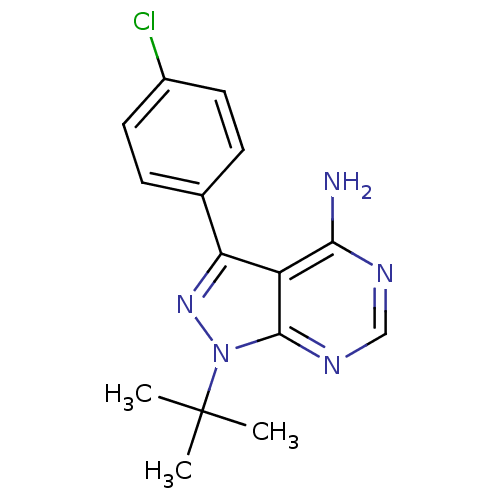
(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50381703
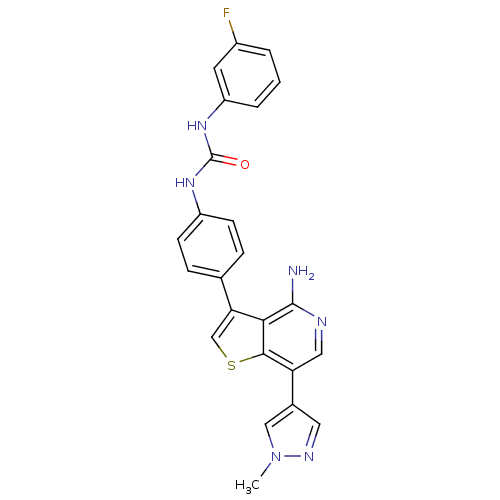
(CHEMBL1970317)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 Show InChI InChI=1S/C24H19FN6OS/c1-31-12-15(10-28-31)19-11-27-23(26)21-20(13-33-22(19)21)14-5-7-17(8-6-14)29-24(32)30-18-4-2-3-16(25)9-18/h2-13H,1H3,(H2,26,27)(H2,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50388873
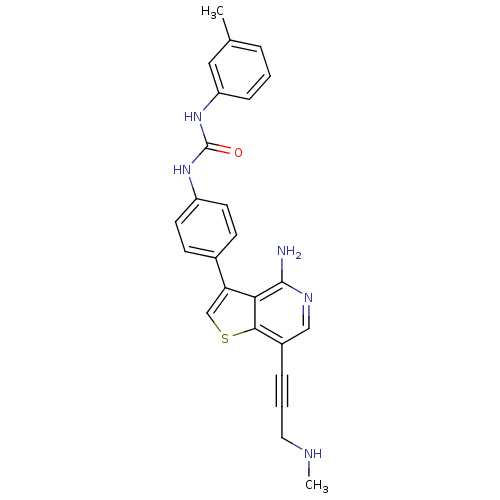
(CHEMBL1964441)Show SMILES CNCC#Cc1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 Show InChI InChI=1S/C25H23N5OS/c1-16-5-3-7-20(13-16)30-25(31)29-19-10-8-17(9-11-19)21-15-32-23-18(6-4-12-27-2)14-28-24(26)22(21)23/h3,5,7-11,13-15,27H,12H2,1-2H3,(H2,26,28)(H2,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRA |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50381719

(CHEMBL2022856)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 |r| Show InChI InChI=1S/C26H23FN6O2S/c1-15(34)12-33-13-17(10-30-33)21-11-29-25(28)23-22(14-36-24(21)23)16-5-7-19(8-6-16)31-26(35)32-20-4-2-3-18(27)9-20/h2-11,13-15,34H,12H2,1H3,(H2,28,29)(H2,31,32,35)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50381716

(ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...)Show SMILES Nc1ncc(-c2cnn(CCO)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50388874
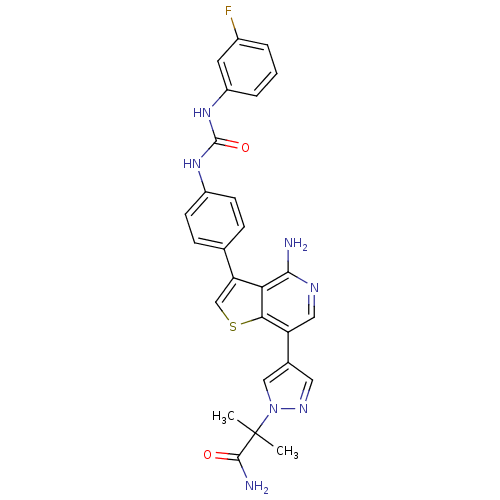
(CHEMBL1967720)Show SMILES CC(C)(C(N)=O)n1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 Show InChI InChI=1S/C27H24FN7O2S/c1-27(2,25(30)36)35-13-16(11-32-35)20-12-31-24(29)22-21(14-38-23(20)22)15-6-8-18(9-7-15)33-26(37)34-19-5-3-4-17(28)10-19/h3-14H,1-2H3,(H2,29,31)(H2,30,36)(H2,33,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50388873

(CHEMBL1964441)Show SMILES CNCC#Cc1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 Show InChI InChI=1S/C25H23N5OS/c1-16-5-3-7-20(13-16)30-25(31)29-19-10-8-17(9-11-19)21-15-32-23-18(6-4-12-27-2)14-28-24(26)22(21)23/h3,5,7-11,13-15,27H,12H2,1-2H3,(H2,26,28)(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50388878

(CHEMBL1999931 | US9163007, 73)Show SMILES O=C(Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1)c1ccccc1 Show InChI InChI=1S/C23H18N6O/c30-23(17-9-5-2-6-10-17)24-22-19-13-18(11-12-20(19)25-27-22)21-15-29(28-26-21)14-16-7-3-1-4-8-16/h1-13,15H,14H2,(H2,24,25,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50388880
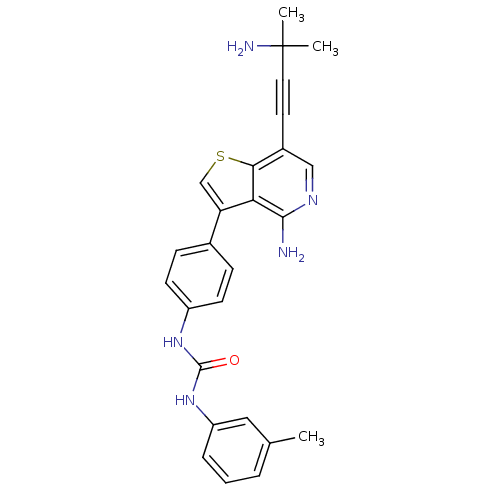
(CHEMBL1979577)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2csc3c(cnc(N)c23)C#CC(C)(C)N)c1 Show InChI InChI=1S/C26H25N5OS/c1-16-5-4-6-20(13-16)31-25(32)30-19-9-7-17(8-10-19)21-15-33-23-18(11-12-26(2,3)28)14-29-24(27)22(21)23/h4-10,13-15H,28H2,1-3H3,(H2,27,29)(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50388875
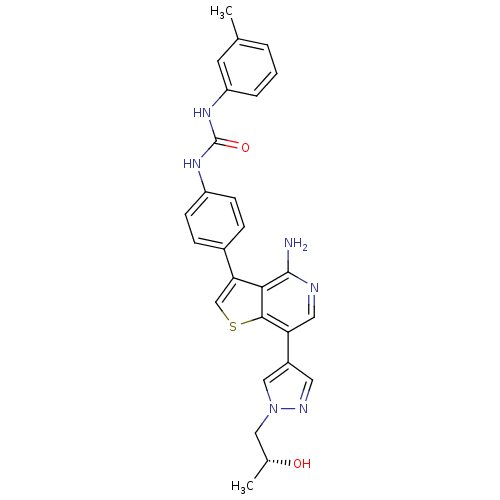
(CHEMBL2062935)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C27H26N6O2S/c1-16-4-3-5-21(10-16)32-27(35)31-20-8-6-18(7-9-20)23-15-36-25-22(12-29-26(28)24(23)25)19-11-30-33(14-19)13-17(2)34/h3-12,14-15,17,34H,13H2,1-2H3,(H2,28,29)(H2,31,32,35)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KIT |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50315893
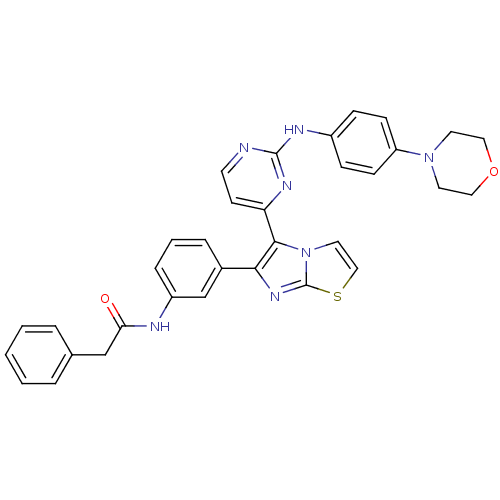
(CHEMBL1090356 | N-(3-(5-(2-(4-morpholinophenylamin...)Show SMILES O=C(Cc1ccccc1)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C33H29N7O2S/c41-29(21-23-5-2-1-3-6-23)35-26-8-4-7-24(22-26)30-31(40-17-20-43-33(40)38-30)28-13-14-34-32(37-28)36-25-9-11-27(12-10-25)39-15-18-42-19-16-39/h1-14,17,20,22H,15-16,18-19,21H2,(H,35,41)(H,34,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50388877

(CHEMBL2062936)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cl)cc(n1)-c1c[nH]c2ncccc12 |r,wU:4.7,wD:1.0,(29.96,-30.16,;29.85,-28.62,;29.57,-27.1,;27.75,-27.13,;26.77,-26.34,;27.05,-27.87,;28.79,-27.81,;25.98,-25.03,;26.73,-23.68,;28.27,-23.66,;29.01,-22.3,;30.55,-22.27,;28.22,-20.99,;26.69,-21.02,;25.94,-22.36,;25.9,-19.71,;24.37,-19.57,;24.02,-18.07,;25.34,-17.28,;25.64,-15.76,;27.1,-15.27,;28.26,-16.29,;27.96,-17.79,;26.5,-18.29,)| Show InChI InChI=1S/C17H18ClN5O/c18-15-8-14(13-9-20-16-12(13)2-1-7-19-16)22-17(23-15)21-10-3-5-11(24)6-4-10/h1-2,7-11,24H,3-6H2,(H,19,20)(H,21,22,23)/t10-,11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50381719

(CHEMBL2022856)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 |r| Show InChI InChI=1S/C26H23FN6O2S/c1-15(34)12-33-13-17(10-30-33)21-11-29-25(28)23-22(14-36-24(21)23)16-5-7-19(8-6-16)31-26(35)32-20-4-2-3-18(27)9-20/h2-11,13-15,34H,12H2,1H3,(H2,28,29)(H2,31,32,35)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of KIT |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50315893

(CHEMBL1090356 | N-(3-(5-(2-(4-morpholinophenylamin...)Show SMILES O=C(Cc1ccccc1)Nc1cccc(c1)-c1nc2sccn2c1-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C33H29N7O2S/c41-29(21-23-5-2-1-3-6-23)35-26-8-4-7-24(22-26)30-31(40-17-20-43-33(40)38-30)28-13-14-34-32(37-28)36-25-9-11-27(12-10-25)39-15-18-42-19-16-39/h1-14,17,20,22H,15-16,18-19,21H2,(H,35,41)(H,34,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50381703

(CHEMBL1970317)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 Show InChI InChI=1S/C24H19FN6OS/c1-31-12-15(10-28-31)19-11-27-23(26)21-20(13-33-22(19)21)14-5-7-17(8-6-14)29-24(32)30-18-4-2-3-16(25)9-18/h2-13H,1H3,(H2,26,27)(H2,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRA |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM25116
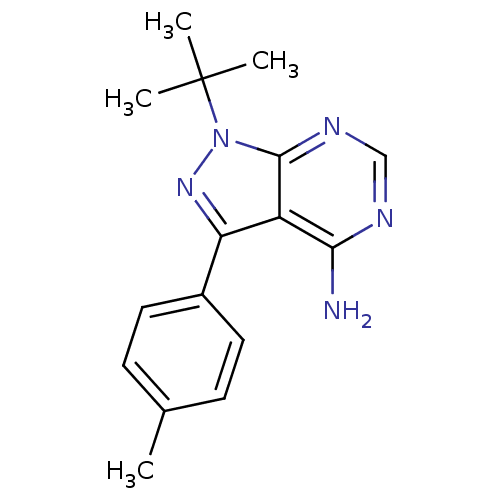
(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50388872
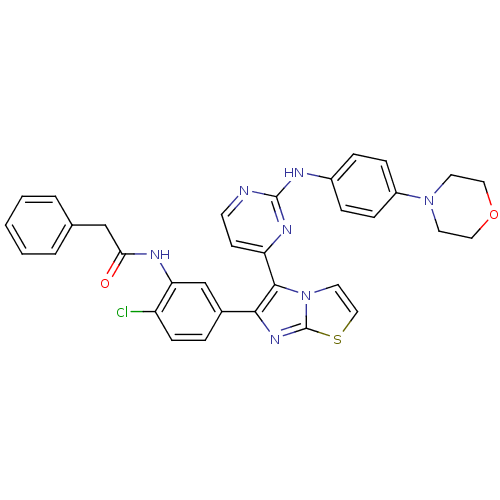
(CHEMBL2062938)Show SMILES Clc1ccc(cc1NC(=O)Cc1ccccc1)-c1nc2sccn2c1-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C33H28ClN7O2S/c34-26-11-6-23(21-28(26)37-29(42)20-22-4-2-1-3-5-22)30-31(41-16-19-44-33(41)39-30)27-12-13-35-32(38-27)36-24-7-9-25(10-8-24)40-14-17-43-18-15-40/h1-13,16,19,21H,14-15,17-18,20H2,(H,37,42)(H,35,36,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM25116

(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50388875

(CHEMBL2062935)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C27H26N6O2S/c1-16-4-3-5-21(10-16)32-27(35)31-20-8-6-18(7-9-20)23-15-36-25-22(12-29-26(28)24(23)25)19-11-30-33(14-19)13-17(2)34/h3-12,14-15,17,34H,13H2,1-2H3,(H2,28,29)(H2,31,32,35)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50388880

(CHEMBL1979577)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2csc3c(cnc(N)c23)C#CC(C)(C)N)c1 Show InChI InChI=1S/C26H25N5OS/c1-16-5-4-6-20(13-16)31-25(32)30-19-9-7-17(8-10-19)21-15-33-23-18(11-12-26(2,3)28)14-29-24(27)22(21)23/h4-10,13-15H,28H2,1-3H3,(H2,27,29)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRA |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50388876

(CHEMBL1983595)Show SMILES Clc1cccc(c1)C(=O)Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1 Show InChI InChI=1S/C23H17ClN6O/c24-18-8-4-7-17(11-18)23(31)25-22-19-12-16(9-10-20(19)26-28-22)21-14-30(29-27-21)13-15-5-2-1-3-6-15/h1-12,14H,13H2,(H2,25,26,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50388879

(CHEMBL2062937)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)Nc1nc(Cl)cc(n1)-c1c[nH]c2ncccc12 |r,wU:6.9,wD:3.2,(39.49,-29.31,;40.58,-28.23,;42.07,-28.64,;40.19,-26.75,;39.91,-25.24,;38.09,-25.26,;37.12,-24.48,;37.39,-26,;39.14,-25.95,;36.32,-23.16,;37.07,-21.81,;38.61,-21.79,;39.36,-20.43,;40.9,-20.4,;38.56,-19.12,;37.03,-19.15,;36.28,-20.5,;36.25,-17.83,;34.71,-17.7,;34.37,-16.2,;35.69,-15.41,;35.99,-13.89,;37.45,-13.39,;38.6,-14.42,;38.3,-15.92,;36.85,-16.42,)| Show InChI InChI=1S/C19H23ClN6/c1-26(2)13-7-5-12(6-8-13)23-19-24-16(10-17(20)25-19)15-11-22-18-14(15)4-3-9-21-18/h3-4,9-13H,5-8H2,1-2H3,(H,21,22)(H,23,24,25)/t12-,13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50388874

(CHEMBL1967720)Show SMILES CC(C)(C(N)=O)n1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 Show InChI InChI=1S/C27H24FN7O2S/c1-27(2,25(30)36)35-13-16(11-32-35)20-12-31-24(29)22-21(14-38-23(20)22)15-6-8-18(9-7-15)33-26(37)34-19-5-3-4-17(28)10-19/h3-14H,1-2H3,(H2,29,31)(H2,30,36)(H2,33,34,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRA |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50381719

(CHEMBL2022856)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 |r| Show InChI InChI=1S/C26H23FN6O2S/c1-15(34)12-33-13-17(10-30-33)21-11-29-25(28)23-22(14-36-24(21)23)16-5-7-19(8-6-16)31-26(35)32-20-4-2-3-18(27)9-20/h2-11,13-15,34H,12H2,1H3,(H2,28,29)(H2,31,32,35)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRA |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50388872

(CHEMBL2062938)Show SMILES Clc1ccc(cc1NC(=O)Cc1ccccc1)-c1nc2sccn2c1-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C33H28ClN7O2S/c34-26-11-6-23(21-28(26)37-29(42)20-22-4-2-1-3-5-22)30-31(41-16-19-44-33(41)39-30)27-12-13-35-32(38-27)36-24-7-9-25(10-8-24)40-14-17-43-18-15-40/h1-13,16,19,21H,14-15,17-18,20H2,(H,37,42)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data