 Found 113 hits Enz. Inhib. hit(s) with all data for entry = 50040106
Found 113 hits Enz. Inhib. hit(s) with all data for entry = 50040106 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389234
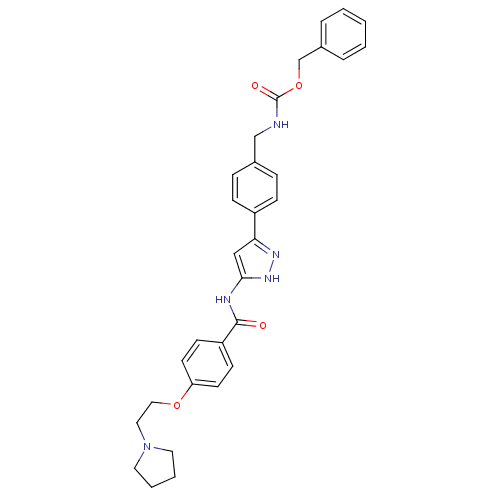
(CHEMBL2063324)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O4/c37-30(26-12-14-27(15-13-26)39-19-18-36-16-4-5-17-36)33-29-20-28(34-35-29)25-10-8-23(9-11-25)21-32-31(38)40-22-24-6-2-1-3-7-24/h1-3,6-15,20H,4-5,16-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 autophosphorylation in human MV4-11 cells after 2 hrs by Western blot analysis |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389222
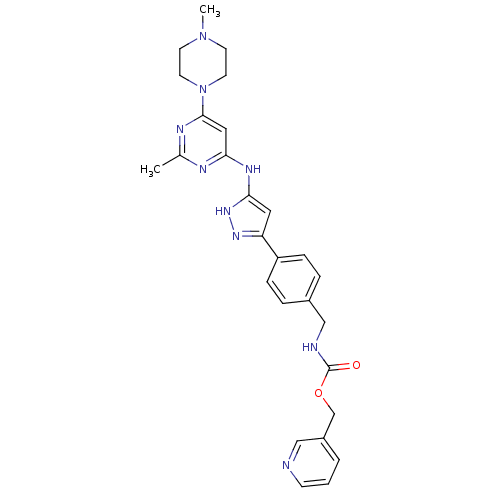
(CHEMBL2063336)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3cccnc3)cc2)nc(C)n1 Show InChI InChI=1S/C27H31N9O2/c1-19-30-24(15-26(31-19)36-12-10-35(2)11-13-36)32-25-14-23(33-34-25)22-7-5-20(6-8-22)17-29-27(37)38-18-21-4-3-9-28-16-21/h3-9,14-16H,10-13,17-18H2,1-2H3,(H,29,37)(H2,30,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389223
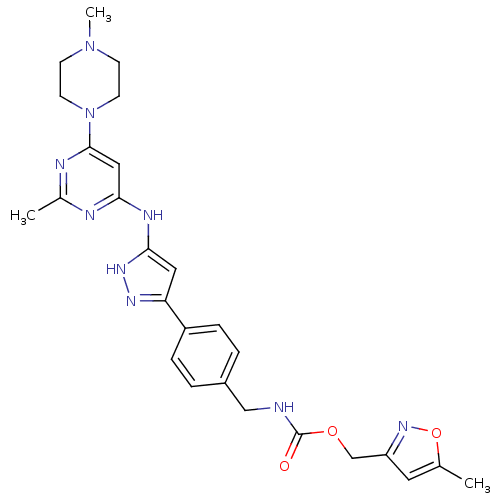
(CHEMBL2063337)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3cc(C)on3)cc2)nc(C)n1 Show InChI InChI=1S/C26H31N9O3/c1-17-12-21(33-38-17)16-37-26(36)27-15-19-4-6-20(7-5-19)22-13-24(32-31-22)30-23-14-25(29-18(2)28-23)35-10-8-34(3)9-11-35/h4-7,12-14H,8-11,15-16H2,1-3H3,(H,27,36)(H2,28,29,30,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389233
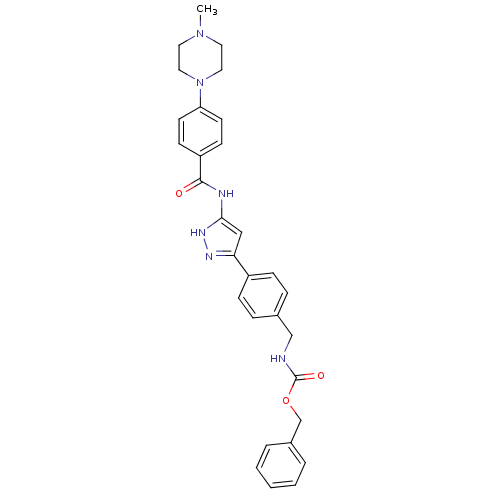
(CHEMBL2063323)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C30H32N6O3/c1-35-15-17-36(18-16-35)26-13-11-25(12-14-26)29(37)32-28-19-27(33-34-28)24-9-7-22(8-10-24)20-31-30(38)39-21-23-5-3-2-4-6-23/h2-14,19H,15-18,20-21H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389228

(CHEMBL2063319)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)25-13-9-23(10-14-25)28(36)31-27-19-26(32-33-27)22-7-11-24(12-8-22)30-29(37)38-20-21-5-3-2-4-6-21/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21079
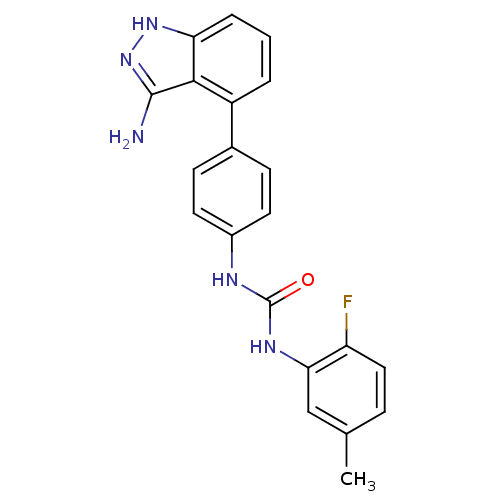
(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389235
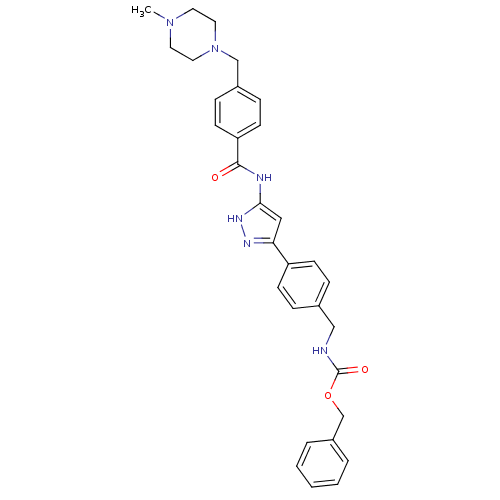
(CHEMBL2063325)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-36-15-17-37(18-16-36)21-24-9-13-27(14-10-24)30(38)33-29-19-28(34-35-29)26-11-7-23(8-12-26)20-32-31(39)40-22-25-5-3-2-4-6-25/h2-14,19H,15-18,20-22H2,1H3,(H,32,39)(H2,33,34,35,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389234

(CHEMBL2063324)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O4/c37-30(26-12-14-27(15-13-26)39-19-18-36-16-4-5-17-36)33-29-20-28(34-35-29)25-10-8-23(9-11-25)21-32-31(38)40-22-24-6-2-1-3-7-24/h1-3,6-15,20H,4-5,16-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389224

(CHEMBL2063338)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C29H34N8O3/c1-21-31-26(18-28(32-21)37-13-11-36(12-14-37)15-16-38)33-27-17-25(34-35-27)24-9-7-22(8-10-24)19-30-29(39)40-20-23-5-3-2-4-6-23/h2-10,17-18,38H,11-16,19-20H2,1H3,(H,30,39)(H2,31,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389229

(CHEMBL2063318)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C28H28N6O3/c1-33-15-17-34(18-16-33)23-13-9-21(10-14-23)27(35)30-26-19-25(31-32-26)20-7-11-22(12-8-20)29-28(36)37-24-5-3-2-4-6-24/h2-14,19H,15-18H2,1H3,(H,29,36)(H2,30,31,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389214
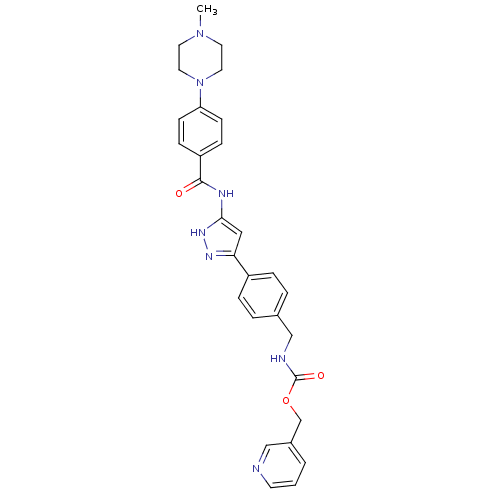
(CHEMBL2063328)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2cccnc2)cc1 Show InChI InChI=1S/C29H31N7O3/c1-35-13-15-36(16-14-35)25-10-8-24(9-11-25)28(37)32-27-17-26(33-34-27)23-6-4-21(5-7-23)19-31-29(38)39-20-22-3-2-12-30-18-22/h2-12,17-18H,13-16,19-20H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389231

(CHEMBL2063321)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)24-13-11-23(12-14-24)28(36)31-27-19-26(32-33-27)22-9-7-21(8-10-22)20-30-29(37)38-25-5-3-2-4-6-25/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389227

(CHEMBL2063436)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)cc(n1)N1CCNCC1 Show InChI InChI=1S/C27H30N8O2/c1-19-30-24(16-26(31-19)35-13-11-28-12-14-35)32-25-15-23(33-34-25)22-9-7-20(8-10-22)17-29-27(36)37-18-21-5-3-2-4-6-21/h2-10,15-16,28H,11-14,17-18H2,1H3,(H,29,36)(H2,30,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389232

(CHEMBL2063322)Show SMILES CCOC(=O)NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(cc2)N2CCN(C)CC2)[nH]n1 Show InChI InChI=1S/C25H30N6O3/c1-3-34-25(33)26-17-18-4-6-19(7-5-18)22-16-23(29-28-22)27-24(32)20-8-10-21(11-9-20)31-14-12-30(2)13-15-31/h4-11,16H,3,12-15,17H2,1-2H3,(H,26,33)(H2,27,28,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389236
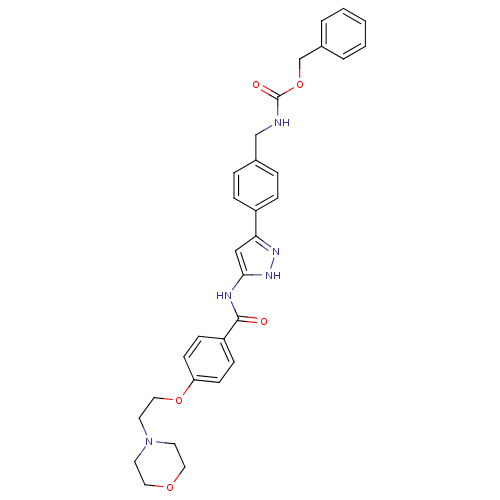
(CHEMBL2063326)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCOCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O5/c37-30(26-10-12-27(13-11-26)40-19-16-36-14-17-39-18-15-36)33-29-20-28(34-35-29)25-8-6-23(7-9-25)21-32-31(38)41-22-24-4-2-1-3-5-24/h1-13,20H,14-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389221
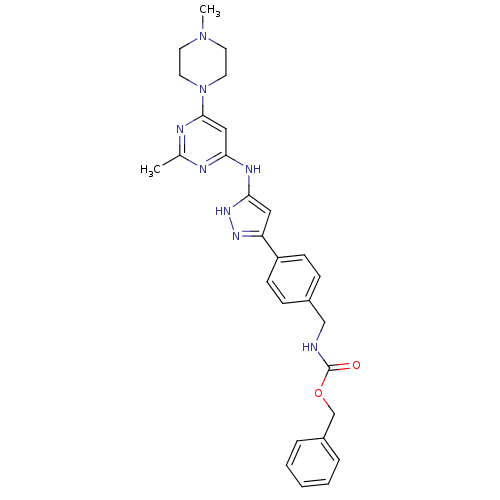
(CHEMBL2063335)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)nc(C)n1 Show InChI InChI=1S/C28H32N8O2/c1-20-30-25(17-27(31-20)36-14-12-35(2)13-15-36)32-26-16-24(33-34-26)23-10-8-21(9-11-23)18-29-28(37)38-19-22-6-4-3-5-7-22/h3-11,16-17H,12-15,18-19H2,1-2H3,(H,29,37)(H2,30,31,32,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389237
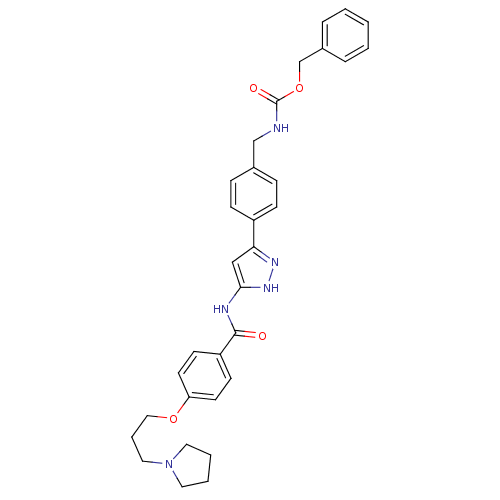
(CHEMBL2063327)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C32H35N5O4/c38-31(27-13-15-28(16-14-27)40-20-6-19-37-17-4-5-18-37)34-30-21-29(35-36-30)26-11-9-24(10-12-26)22-33-32(39)41-23-25-7-2-1-3-8-25/h1-3,7-16,21H,4-6,17-20,22-23H2,(H,33,39)(H2,34,35,36,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389233

(CHEMBL2063323)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C30H32N6O3/c1-35-15-17-36(18-16-35)26-13-11-25(12-14-26)29(37)32-28-19-27(33-34-28)24-9-7-22(8-10-24)20-31-30(38)39-21-23-5-3-2-4-6-23/h2-14,19H,15-18,20-21H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389216

(CHEMBL2063330)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCOCC3)cc2)[nH]n1)OCc1cccnc1 Show InChI InChI=1S/C30H32N6O5/c37-29(25-7-9-26(10-8-25)40-17-14-36-12-15-39-16-13-36)33-28-18-27(34-35-28)24-5-3-22(4-6-24)20-32-30(38)41-21-23-2-1-11-31-19-23/h1-11,18-19H,12-17,20-21H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389225
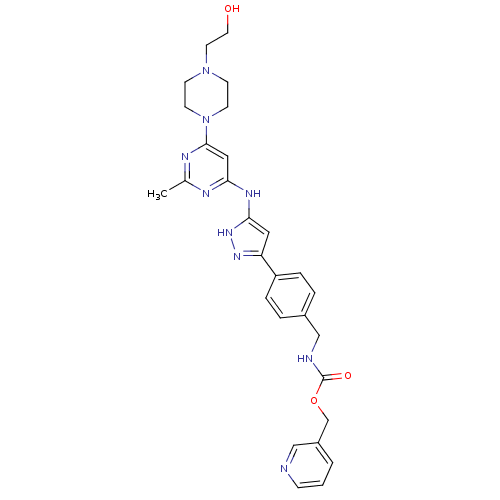
(CHEMBL2063434)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3cccnc3)cc2)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C28H33N9O3/c1-20-31-25(16-27(32-20)37-11-9-36(10-12-37)13-14-38)33-26-15-24(34-35-26)23-6-4-21(5-7-23)18-30-28(39)40-19-22-3-2-8-29-17-22/h2-8,15-17,38H,9-14,18-19H2,1H3,(H,30,39)(H2,31,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389217

(CHEMBL2063331)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2cc(C)on2)cc1 Show InChI InChI=1S/C28H31N7O4/c1-19-15-23(33-39-19)18-38-28(37)29-17-20-3-5-21(6-4-20)25-16-26(32-31-25)30-27(36)22-7-9-24(10-8-22)35-13-11-34(2)12-14-35/h3-10,15-16H,11-14,17-18H2,1-2H3,(H,29,37)(H2,30,31,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389215
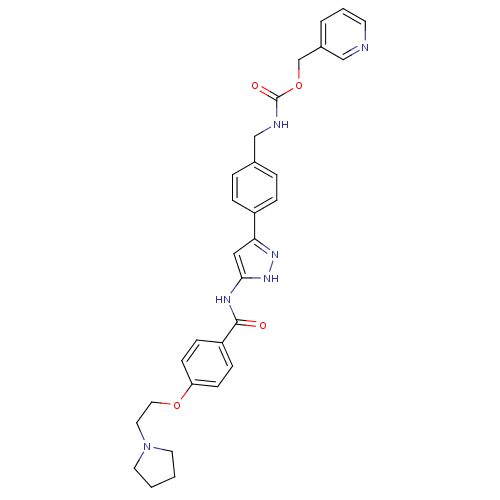
(CHEMBL2063329)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1cccnc1 Show InChI InChI=1S/C30H32N6O4/c37-29(25-9-11-26(12-10-25)39-17-16-36-14-1-2-15-36)33-28-18-27(34-35-28)24-7-5-22(6-8-24)20-32-30(38)40-21-23-4-3-13-31-19-23/h3-13,18-19H,1-2,14-17,20-21H2,(H,32,38)(H2,33,34,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389230
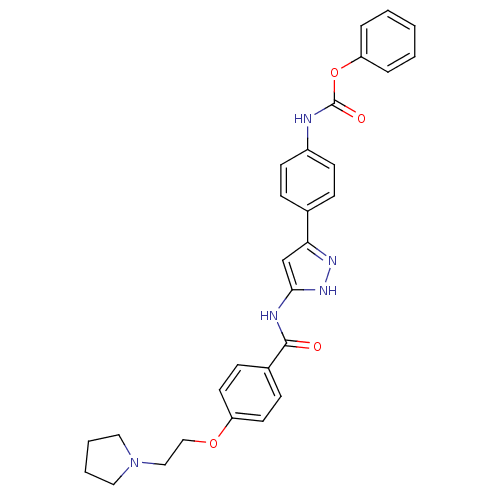
(CHEMBL2063320)Show SMILES O=C(Nc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)Oc1ccccc1 Show InChI InChI=1S/C29H29N5O4/c35-28(22-10-14-24(15-11-22)37-19-18-34-16-4-5-17-34)31-27-20-26(32-33-27)21-8-12-23(13-9-21)30-29(36)38-25-6-2-1-3-7-25/h1-3,6-15,20H,4-5,16-19H2,(H,30,36)(H2,31,32,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389218
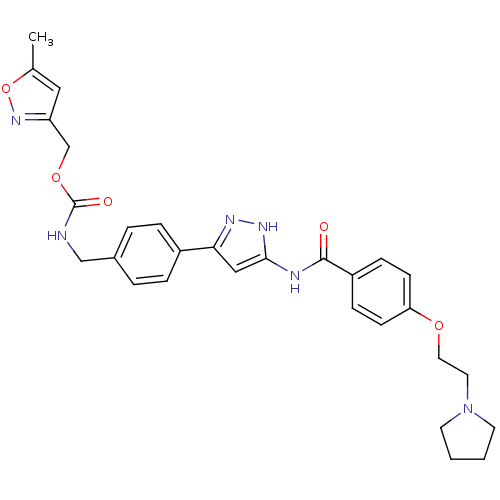
(CHEMBL2063332)Show SMILES Cc1cc(COC(=O)NCc2ccc(cc2)-c2cc(NC(=O)c3ccc(OCCN4CCCC4)cc3)[nH]n2)no1 Show InChI InChI=1S/C29H32N6O5/c1-20-16-24(34-40-20)19-39-29(37)30-18-21-4-6-22(7-5-21)26-17-27(33-32-26)31-28(36)23-8-10-25(11-9-23)38-15-14-35-12-2-3-13-35/h4-11,16-17H,2-3,12-15,18-19H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389228

(CHEMBL2063319)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)25-13-9-23(10-14-25)28(36)31-27-19-26(32-33-27)22-7-11-24(12-8-22)30-29(37)38-20-21-5-3-2-4-6-21/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389219
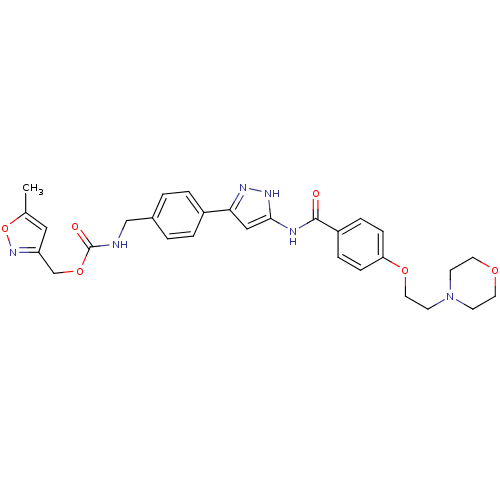
(CHEMBL2063333)Show SMILES Cc1cc(COC(=O)NCc2ccc(cc2)-c2cc(NC(=O)c3ccc(OCCN4CCOCC4)cc3)[nH]n2)no1 Show InChI InChI=1S/C29H32N6O6/c1-20-16-24(34-41-20)19-40-29(37)30-18-21-2-4-22(5-3-21)26-17-27(33-32-26)31-28(36)23-6-8-25(9-7-23)39-15-12-35-10-13-38-14-11-35/h2-9,16-17H,10-15,18-19H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389231

(CHEMBL2063321)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)24-13-11-23(12-14-24)28(36)31-27-19-26(32-33-27)22-9-7-21(8-10-22)20-30-29(37)38-25-5-3-2-4-6-25/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389234

(CHEMBL2063324)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O4/c37-30(26-12-14-27(15-13-26)39-19-18-36-16-4-5-17-36)33-29-20-28(34-35-29)25-10-8-23(9-11-25)21-32-31(38)40-22-24-6-2-1-3-7-24/h1-3,6-15,20H,4-5,16-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389226
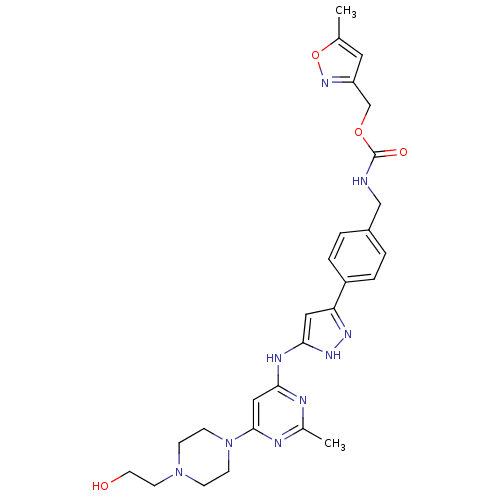
(CHEMBL2063435)Show SMILES Cc1cc(COC(=O)NCc2ccc(cc2)-c2cc(Nc3cc(nc(C)n3)N3CCN(CCO)CC3)[nH]n2)no1 Show InChI InChI=1S/C27H33N9O4/c1-18-13-22(34-40-18)17-39-27(38)28-16-20-3-5-21(6-4-20)23-14-25(33-32-23)31-24-15-26(30-19(2)29-24)36-9-7-35(8-10-36)11-12-37/h3-6,13-15,37H,7-12,16-17H2,1-2H3,(H,28,38)(H2,29,30,31,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389227

(CHEMBL2063436)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)cc(n1)N1CCNCC1 Show InChI InChI=1S/C27H30N8O2/c1-19-30-24(16-26(31-19)35-13-11-28-12-14-35)32-25-15-23(33-34-25)22-9-7-20(8-10-22)17-29-27(36)37-18-21-5-3-2-4-6-21/h2-10,15-16,28H,11-14,17-18H2,1H3,(H,29,36)(H2,30,31,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389231

(CHEMBL2063321)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)24-13-11-23(12-14-24)28(36)31-27-19-26(32-33-27)22-9-7-21(8-10-22)20-30-29(37)38-25-5-3-2-4-6-25/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389233

(CHEMBL2063323)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C30H32N6O3/c1-35-15-17-36(18-16-35)26-13-11-25(12-14-26)29(37)32-28-19-27(33-34-28)24-9-7-22(8-10-24)20-31-30(38)39-21-23-5-3-2-4-6-23/h2-14,19H,15-18,20-21H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389234

(CHEMBL2063324)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C31H33N5O4/c37-30(26-12-14-27(15-13-26)39-19-18-36-16-4-5-17-36)33-29-20-28(34-35-29)25-10-8-23(9-11-25)21-32-31(38)40-22-24-6-2-1-3-7-24/h1-3,6-15,20H,4-5,16-19,21-22H2,(H,32,38)(H2,33,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50389220
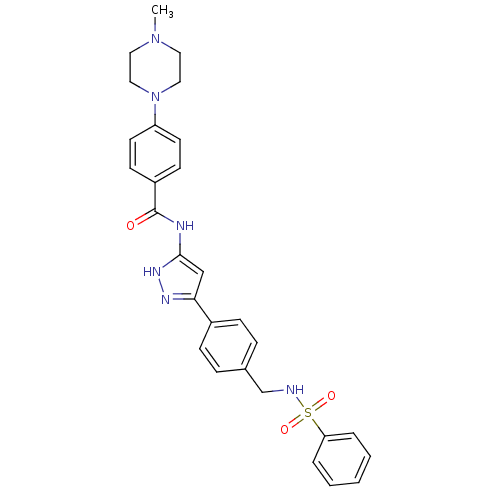
(CHEMBL2063334)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C28H30N6O3S/c1-33-15-17-34(18-16-33)24-13-11-23(12-14-24)28(35)30-27-19-26(31-32-27)22-9-7-21(8-10-22)20-29-38(36,37)25-5-3-2-4-6-25/h2-14,19,29H,15-18,20H2,1H3,(H2,30,31,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of wild type FLT3 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50389224

(CHEMBL2063338)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C29H34N8O3/c1-21-31-26(18-28(32-21)37-13-11-36(12-14-37)15-16-38)33-27-17-25(34-35-27)24-9-7-22(8-10-24)19-30-29(39)40-20-23-5-3-2-4-6-23/h2-10,17-18,38H,11-16,19-20H2,1H3,(H,30,39)(H2,31,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389235

(CHEMBL2063325)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C31H34N6O3/c1-36-15-17-37(18-16-36)21-24-9-13-27(14-10-24)30(38)33-29-19-28(34-35-29)26-11-7-23(8-12-26)20-32-31(39)40-22-25-5-3-2-4-6-25/h2-14,19H,15-18,20-22H2,1H3,(H,32,39)(H2,33,34,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389229

(CHEMBL2063318)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)Oc2ccccc2)cc1 Show InChI InChI=1S/C28H28N6O3/c1-33-15-17-34(18-16-33)23-13-9-21(10-14-23)27(35)30-26-19-25(31-32-26)20-7-11-22(12-8-20)29-28(36)37-24-5-3-2-4-6-24/h2-14,19H,15-18H2,1H3,(H,29,36)(H2,30,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389237

(CHEMBL2063327)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C32H35N5O4/c38-31(27-13-15-28(16-14-27)40-20-6-19-37-17-4-5-18-37)34-30-21-29(35-36-30)26-11-9-24(10-12-26)22-33-32(39)41-23-25-7-2-1-3-8-25/h1-3,7-16,21H,4-6,17-20,22-23H2,(H,33,39)(H2,34,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389227

(CHEMBL2063436)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)cc(n1)N1CCNCC1 Show InChI InChI=1S/C27H30N8O2/c1-19-30-24(16-26(31-19)35-13-11-28-12-14-35)32-25-15-23(33-34-25)22-9-7-20(8-10-22)17-29-27(36)37-18-21-5-3-2-4-6-21/h2-10,15-16,28H,11-14,17-18H2,1H3,(H,29,36)(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389228

(CHEMBL2063319)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C29H30N6O3/c1-34-15-17-35(18-16-34)25-13-9-23(10-14-25)28(36)31-27-19-26(32-33-27)22-7-11-24(12-8-22)30-29(37)38-20-21-5-3-2-4-6-21/h2-14,19H,15-18,20H2,1H3,(H,30,37)(H2,31,32,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389214

(CHEMBL2063328)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2cccnc2)cc1 Show InChI InChI=1S/C29H31N7O3/c1-35-13-15-36(16-14-35)25-10-8-24(9-11-25)28(37)32-27-17-26(33-34-27)23-6-4-21(5-7-23)19-31-29(38)39-20-22-3-2-12-30-18-22/h2-12,17-18H,13-16,19-20H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389237

(CHEMBL2063327)Show SMILES O=C(NCc1ccc(cc1)-c1cc(NC(=O)c2ccc(OCCCN3CCCC3)cc2)[nH]n1)OCc1ccccc1 Show InChI InChI=1S/C32H35N5O4/c38-31(27-13-15-28(16-14-27)40-20-6-19-37-17-4-5-18-37)34-30-21-29(35-36-30)26-11-9-24(10-12-26)22-33-32(39)41-23-25-7-2-1-3-8-25/h1-3,7-16,21H,4-6,17-20,22-23H2,(H,33,39)(H2,34,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50389224

(CHEMBL2063338)Show SMILES Cc1nc(Nc2cc(n[nH]2)-c2ccc(CNC(=O)OCc3ccccc3)cc2)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C29H34N8O3/c1-21-31-26(18-28(32-21)37-13-11-36(12-14-37)15-16-38)33-27-17-25(34-35-27)24-9-7-22(8-10-24)19-30-29(39)40-20-23-5-3-2-4-6-23/h2-10,17-18,38H,11-16,19-20H2,1H3,(H,30,39)(H2,31,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50389214

(CHEMBL2063328)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1cc(n[nH]1)-c1ccc(CNC(=O)OCc2cccnc2)cc1 Show InChI InChI=1S/C29H31N7O3/c1-35-13-15-36(16-14-35)25-10-8-24(9-11-25)28(37)32-27-17-26(33-34-27)23-6-4-21(5-7-23)19-31-29(38)39-20-22-3-2-12-30-18-22/h2-12,17-18H,13-16,19-20H2,1H3,(H,31,38)(H2,32,33,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 22: 4654-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.116
BindingDB Entry DOI: 10.7270/Q2XS5WGV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data