 Found 47 hits Enz. Inhib. hit(s) with all data for entry = 50040332
Found 47 hits Enz. Inhib. hit(s) with all data for entry = 50040332 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247054
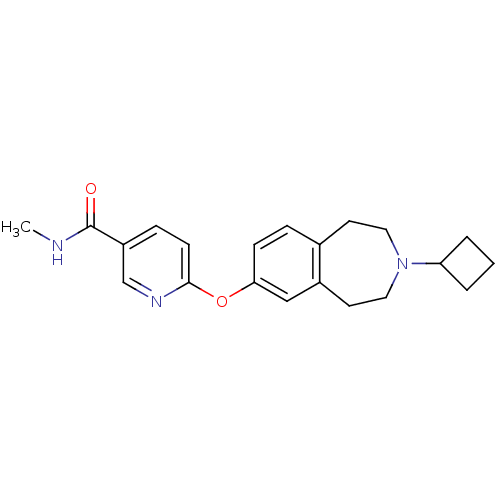
(6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)17-6-8-20(23-14-17)26-19-7-5-15-9-11-24(18-3-2-4-18)12-10-16(15)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50392760
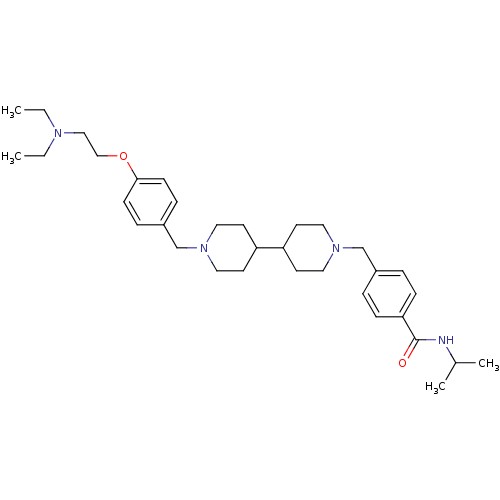
(CHEMBL2151158)Show SMILES CCN(CC)CCOc1ccc(CN2CCC(CC2)C2CCN(Cc3ccc(cc3)C(=O)NC(C)C)CC2)cc1 Show InChI InChI=1S/C34H52N4O2/c1-5-36(6-2)23-24-40-33-13-9-29(10-14-33)26-38-21-17-31(18-22-38)30-15-19-37(20-16-30)25-28-7-11-32(12-8-28)34(39)35-27(3)4/h7-14,27,30-31H,5-6,15-26H2,1-4H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human muscarinic M2 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50074629
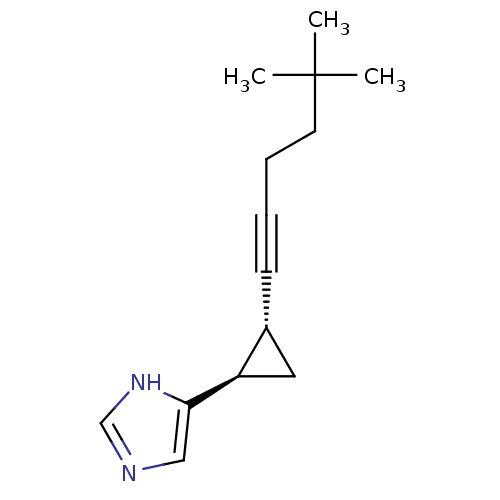
(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200647

((R)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...)Show SMILES C[C@@H]1CCCN1CCc1ccc2cc(ccc2c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24N2/c1-18-3-2-13-26(18)14-12-19-4-9-24-16-23(11-10-22(24)15-19)21-7-5-20(17-25)6-8-21/h4-11,15-16,18H,2-3,12-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224192
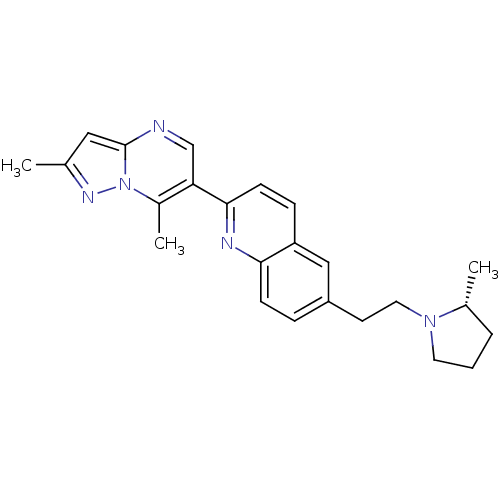
((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnc2cc(C)nn2c1C Show InChI InChI=1S/C24H27N5/c1-16-13-24-25-15-21(18(3)29(24)27-16)23-9-7-20-14-19(6-8-22(20)26-23)10-12-28-11-4-5-17(28)2/h6-9,13-15,17H,4-5,10-12H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50341447
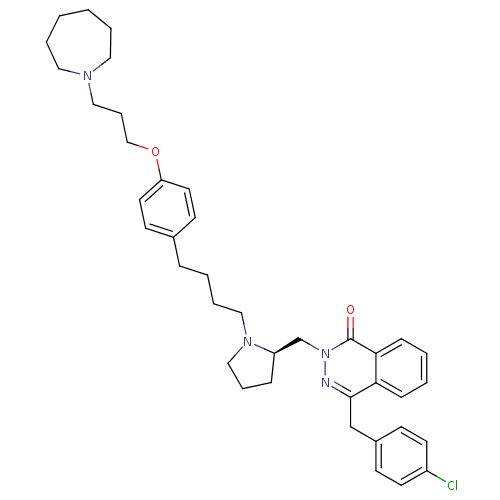
(4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C39H49ClN4O2/c40-33-19-15-32(16-20-33)29-38-36-13-3-4-14-37(36)39(45)44(41-38)30-34-12-9-27-43(34)26-8-5-11-31-17-21-35(22-18-31)46-28-10-25-42-23-6-1-2-7-24-42/h3-4,13-22,34H,1-2,5-12,23-30H2/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392759

(CHEMBL2151157)Show SMILES CC(C)N1CCCN(CC1)C(=O)C1CCN(CC1)c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C20H29F3N4O/c1-15(2)25-8-3-9-27(13-12-25)19(28)16-6-10-26(11-7-16)17-4-5-18(24-14-17)20(21,22)23/h4-5,14-16H,3,6-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392761

(CHEMBL2151155)Show SMILES CS(=O)(=O)c1ccc(cc1)C(=O)N1CCN(CC1)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C27H35N3O4S/c1-35(32,33)26-11-5-21(6-12-26)27(31)30-19-17-29(18-20-30)23-7-9-24(10-8-23)34-25-13-15-28(16-14-25)22-3-2-4-22/h5-12,22,25H,2-4,13-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50370569
(A-349,821 | A-349821)Show SMILES C[C@H]1CC[C@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392763

(CHEMBL2151195)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C18H21N3O/c1-13-3-2-7-21(13)8-6-17-10-15-9-14(4-5-18(15)22-17)16-11-19-20-12-16/h4-5,9-13H,2-3,6-8H2,1H3,(H,19,20)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50247054

(6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)17-6-8-20(23-14-17)26-19-7-5-15-9-11-24(18-3-2-4-18)12-10-16(15)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50158601
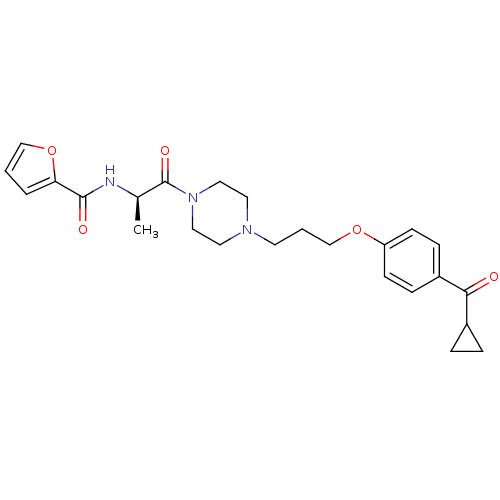
(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392765
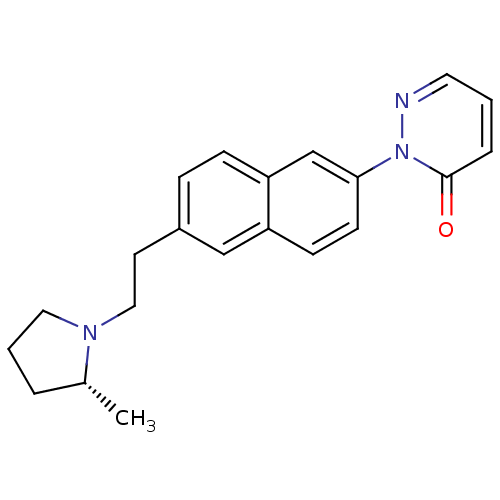
(CHEMBL2151196)Show SMILES C[C@@H]1CCCN1CCc1ccc2cc(ccc2c1)-n1ncccc1=O |r| Show InChI InChI=1S/C21H23N3O/c1-16-4-3-12-23(16)13-10-17-6-7-19-15-20(9-8-18(19)14-17)24-21(25)5-2-11-22-24/h2,5-9,11,14-16H,3-4,10,12-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50224192

((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnc2cc(C)nn2c1C Show InChI InChI=1S/C24H27N5/c1-16-13-24-25-15-21(18(3)29(24)27-16)23-9-7-20-14-19(6-8-22(20)26-23)10-12-28-11-4-5-17(28)2/h6-9,13-15,17H,4-5,10-12H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146835
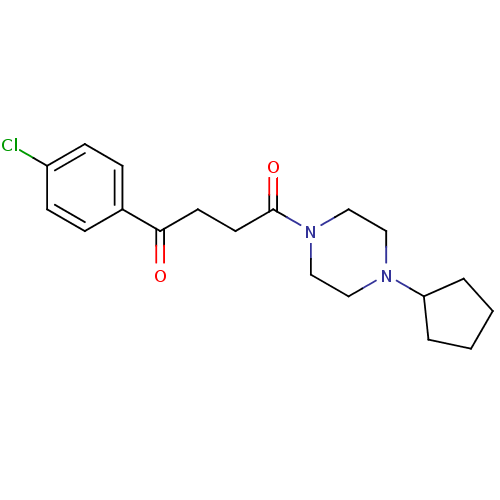
(1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...)Show InChI InChI=1S/C19H25ClN2O2/c20-16-7-5-15(6-8-16)18(23)9-10-19(24)22-13-11-21(12-14-22)17-3-1-2-4-17/h5-8,17H,1-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392764
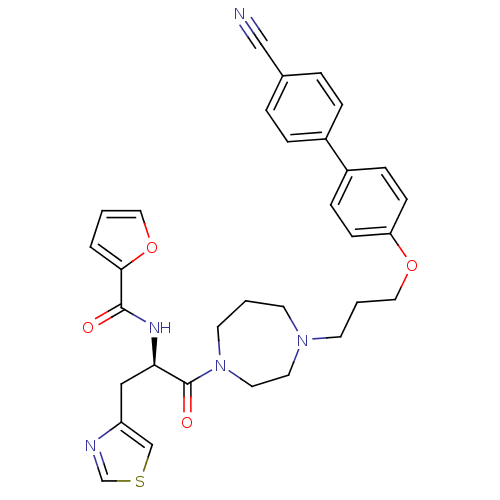
(CHEMBL2151194)Show SMILES O=C(N[C@H](Cc1cscn1)C(=O)N1CCCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)CC1)c1ccco1 |r| Show InChI InChI=1S/C32H33N5O4S/c33-21-24-5-7-25(8-6-24)26-9-11-28(12-10-26)40-19-3-14-36-13-2-15-37(17-16-36)32(39)29(20-27-22-42-23-34-27)35-31(38)30-4-1-18-41-30/h1,4-12,18,22-23,29H,2-3,13-17,19-20H2,(H,35,38)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370569

(A-349,821 | A-349821)Show SMILES C[C@H]1CC[C@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119707
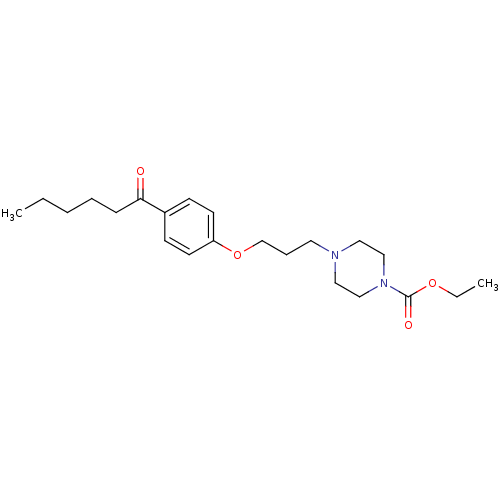
(4-[3-(4-Hexanoyl-phenoxy)-propyl]-piperazine-1-car...)Show InChI InChI=1S/C22H34N2O4/c1-3-5-6-8-21(25)19-9-11-20(12-10-19)28-18-7-13-23-14-16-24(17-15-23)22(26)27-4-2/h9-12H,3-8,13-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543
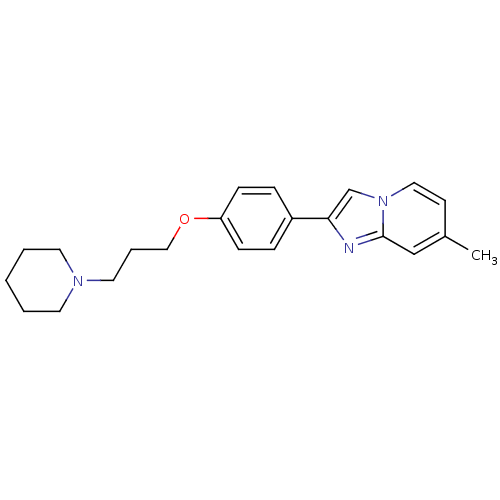
(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50200647

((R)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...)Show SMILES C[C@@H]1CCCN1CCc1ccc2cc(ccc2c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24N2/c1-18-3-2-13-26(18)14-12-19-4-9-24-16-23(11-10-22(24)15-19)21-7-5-20(17-25)6-8-21/h4-11,15-16,18H,2-3,12-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139308
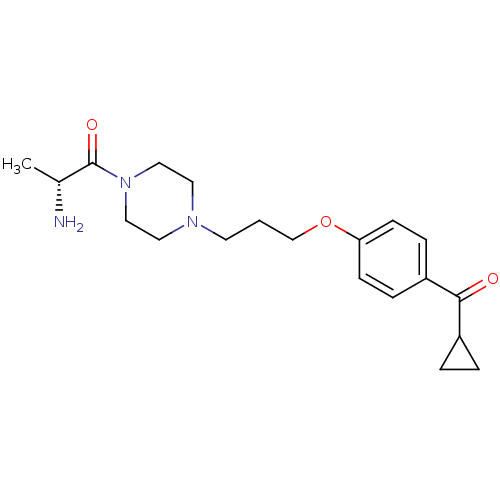
((R)-2-Amino-1-{4-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES C[C@@H](N)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C20H29N3O3/c1-15(21)20(25)23-12-10-22(11-13-23)9-2-14-26-18-7-5-17(6-8-18)19(24)16-3-4-16/h5-8,15-16H,2-4,9-14,21H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50392763

(CHEMBL2151195)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C18H21N3O/c1-13-3-2-7-21(13)8-6-17-10-15-9-14(4-5-18(15)22-17)16-11-19-20-12-16/h4-5,9-13H,2-3,6-8H2,1H3,(H,19,20)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Macaca mulatta (Rhesus macaque)) | BDBM50193199
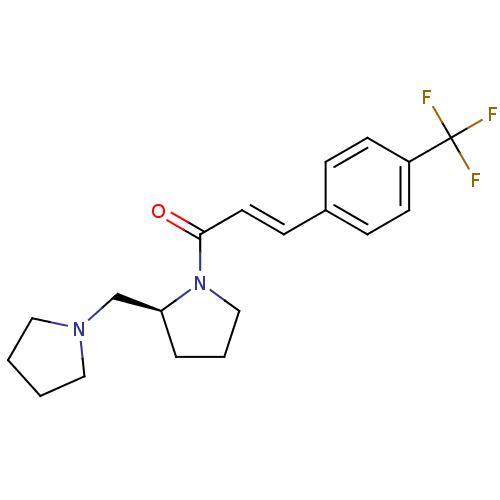
((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 |r| Show InChI InChI=1S/C19H23F3N2O/c20-19(21,22)16-8-5-15(6-9-16)7-10-18(25)24-13-3-4-17(24)14-23-11-1-2-12-23/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rhesus monkey histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193199

((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 |r| Show InChI InChI=1S/C19H23F3N2O/c20-19(21,22)16-8-5-15(6-9-16)7-10-18(25)24-13-3-4-17(24)14-23-11-1-2-12-23/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50128875
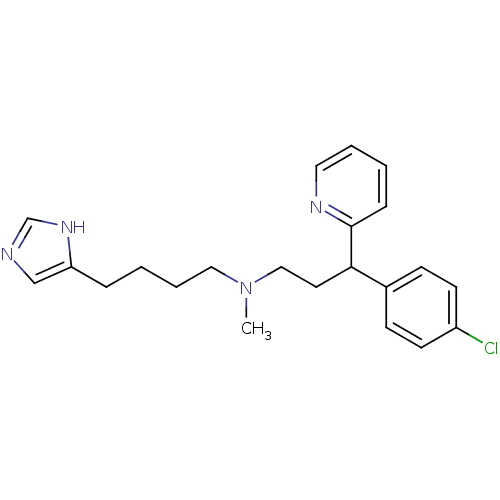
(CHEMBL292195 | [3-(4-Chloro-phenyl)-3-pyridin-2-yl...)Show SMILES CN(CCCCc1cnc[nH]1)CCC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C22H27ClN4/c1-27(14-5-3-6-20-16-24-17-26-20)15-12-21(22-7-2-4-13-25-22)18-8-10-19(23)11-9-18/h2,4,7-11,13,16-17,21H,3,5-6,12,14-15H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50392762
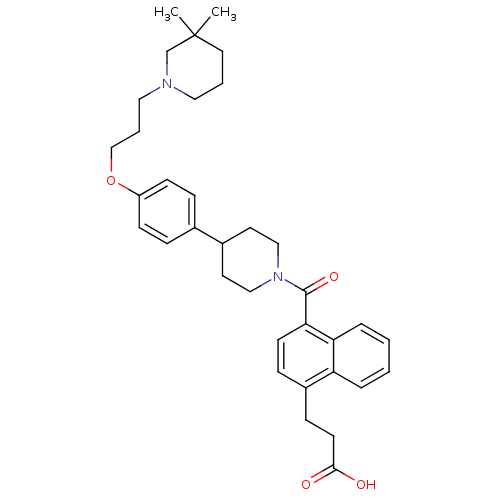
(CHEMBL2151156)Show SMILES CC1(C)CCCN(CCCOc2ccc(cc2)C2CCN(CC2)C(=O)c2ccc(CCC(O)=O)c3ccccc23)C1 Show InChI InChI=1S/C35H44N2O4/c1-35(2)19-5-20-36(25-35)21-6-24-41-29-13-9-26(10-14-29)27-17-22-37(23-18-27)34(40)32-15-11-28(12-16-33(38)39)30-7-3-4-8-31(30)32/h3-4,7-11,13-15,27H,5-6,12,16-25H2,1-2H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50128875

(CHEMBL292195 | [3-(4-Chloro-phenyl)-3-pyridin-2-yl...)Show SMILES CN(CCCCc1cnc[nH]1)CCC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C22H27ClN4/c1-27(14-5-3-6-20-16-24-17-26-20)15-12-21(22-7-2-4-13-25-22)18-8-10-19(23)11-9-18/h2,4,7-11,13,16-17,21H,3,5-6,12,14-15H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to guinea pig histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50341447

(4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C39H49ClN4O2/c40-33-19-15-32(16-20-33)29-38-36-13-3-4-14-37(36)39(45)44(41-38)30-34-12-9-27-43(34)26-8-5-11-31-17-21-35(22-18-31)46-28-10-25-42-23-6-1-2-7-24-42/h3-4,13-22,34H,1-2,5-12,23-30H2/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50392760

(CHEMBL2151158)Show SMILES CCN(CC)CCOc1ccc(CN2CCC(CC2)C2CCN(Cc3ccc(cc3)C(=O)NC(C)C)CC2)cc1 Show InChI InChI=1S/C34H52N4O2/c1-5-36(6-2)23-24-40-33-13-9-29(10-14-33)26-38-21-17-31(18-22-38)30-15-19-37(20-16-30)25-28-7-11-32(12-8-28)34(39)35-27(3)4/h7-14,27,30-31H,5-6,15-26H2,1-4H3,(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to guinea pig histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat histamine H4 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392762

(CHEMBL2151156)Show SMILES CC1(C)CCCN(CCCOc2ccc(cc2)C2CCN(CC2)C(=O)c2ccc(CCC(O)=O)c3ccccc23)C1 Show InChI InChI=1S/C35H44N2O4/c1-35(2)19-5-20-36(25-35)21-6-24-41-29-13-9-26(10-14-29)27-17-22-37(23-18-27)34(40)32-15-11-28(12-16-33(38)39)30-7-3-4-8-31(30)32/h3-4,7-11,13-15,27H,5-6,12,16-25H2,1-2H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158601

(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H4 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139308

((R)-2-Amino-1-{4-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES C[C@@H](N)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C20H29N3O3/c1-15(21)20(25)23-12-10-22(11-13-23)9-2-14-26-18-7-5-17(6-8-18)19(24)16-3-4-16/h5-8,15-16H,2-4,9-14,21H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50392761

(CHEMBL2151155)Show SMILES CS(=O)(=O)c1ccc(cc1)C(=O)N1CCN(CC1)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C27H35N3O4S/c1-35(32,33)26-11-5-21(6-12-26)27(31)30-19-17-29(18-20-30)23-7-9-24(10-8-23)34-25-13-15-28(16-14-25)22-3-2-4-22/h5-12,22,25H,2-4,13-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor by GTPgammaS binding assay |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50392759

(CHEMBL2151157)Show SMILES CC(C)N1CCCN(CC1)C(=O)C1CCN(CC1)c1ccc(nc1)C(F)(F)F Show InChI InChI=1S/C20H29F3N4O/c1-15(2)25-8-3-9-27(13-12-25)19(28)16-6-10-26(11-7-16)17-4-5-18(24-14-17)20(21,22)23/h4-5,14-16H,3,6-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor by GTPgammaS binding assay |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human histamine H4 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human histamine H4 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data