 Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50040549
Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50040549 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041
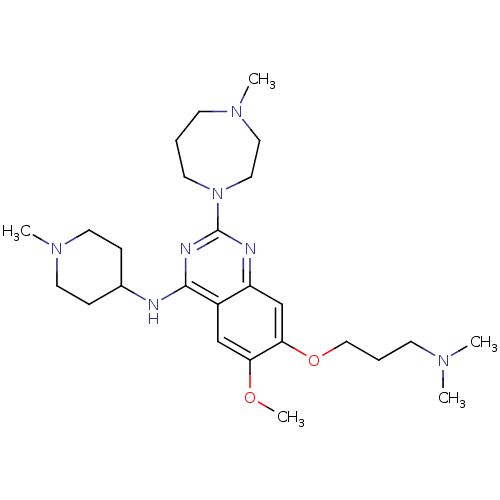
(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant G9a catalytic domain amino acid 913 to 1193 expressed in Escherichia coli BL21 (DE3) by isothermal titration ca... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of G9a by fluorescence polarization assay in presence of fluorescein-labeled H3 peptide |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50396022
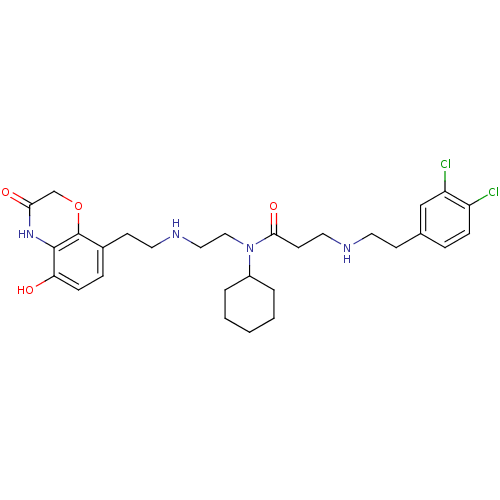
(CHEMBL2169920)Show SMILES Oc1ccc(CCNCCN(C2CCCCC2)C(=O)CCNCCc2ccc(Cl)c(Cl)c2)c2OCC(=O)Nc12 Show InChI InChI=1S/C29H38Cl2N4O4/c30-23-8-6-20(18-24(23)31)10-13-32-15-12-27(38)35(22-4-2-1-3-5-22)17-16-33-14-11-21-7-9-25(36)28-29(21)39-19-26(37)34-28/h6-9,18,22,32-33,36H,1-5,10-17,19H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding affinity to full length human SMYD2 amino acid 1 to 433 expressed in Escherichia coli BL21 (DE3) after 90 mins by radioactive fil... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396023
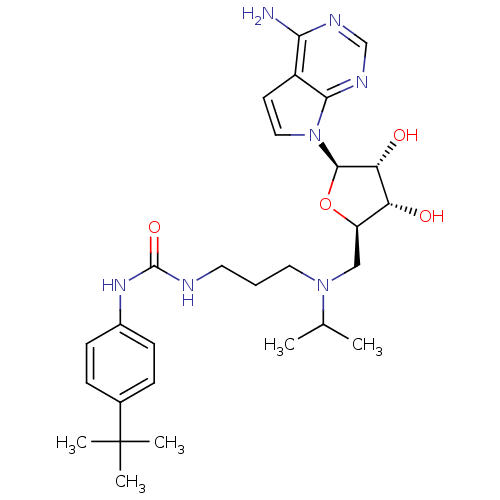
(CHEMBL2169919)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L using [3H]-SAM as substrate after 30 mins |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50396014

(CHEMBL2169888)Show SMILES N[C@@H]1C[C@H]1c1cc(F)cc(F)c1OCc1ccccc1 |r| Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50396015
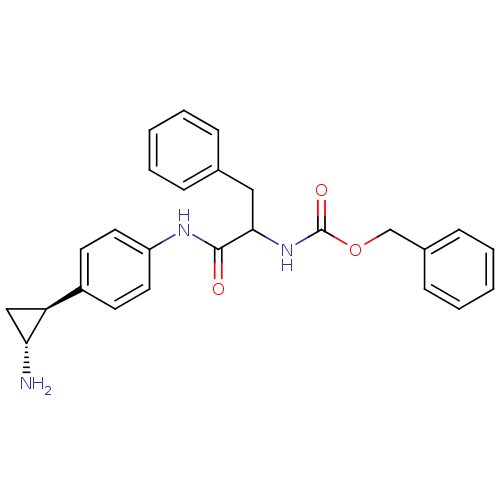
(CHEMBL2169921)Show SMILES N[C@@H]1C[C@H]1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 |r| Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31)/t22-,23+,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 using histone H3 peptide as substrate |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346874

(CHEMBL1797649)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-1-[#6]-[#6]-1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C97H176N36O28/c1-48(2)44-66(87(153)118-52(6)94(160)161)128-84(150)64(32-34-69(102)138)124-79(145)58(23-11-15-37-99)122-82(148)62(27-19-41-112-96(106)107)126-89(155)68-29-21-43-133(68)93(159)51(5)117-78(144)57(22-10-14-36-98)119-72(141)46-114-71(140)45-115-90(156)73(53(7)135)130-88(154)67(47-134)129-83(149)59(24-12-16-38-100)123-81(147)61(26-18-40-111-95(104)105)121-77(143)50(4)116-91(157)74(54(8)136)131-86(152)65(33-35-70(103)139)125-80(146)60(25-13-17-39-110-56-30-31-56)127-92(158)75(55(9)137)132-85(151)63(120-76(142)49(3)101)28-20-42-113-97(108)109/h48-68,73-75,110,134-137H,10-47,98-101H2,1-9H3,(H2,102,138)(H2,103,139)(H,114,140)(H,115,156)(H,116,157)(H,117,144)(H,118,153)(H,119,141)(H,120,142)(H,121,143)(H,122,148)(H,123,147)(H,124,145)(H,125,146)(H,126,155)(H,127,158)(H,128,150)(H,129,149)(H,130,154)(H,131,152)(H,132,151)(H,160,161)(H4,104,105,111)(H4,106,107,112)(H4,108,109,113)/t49-,50-,51-,52-,53+,54+,55+,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,73-,74-,75-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LSD1 catalytic domain amino acid 178 to 831 expressed in Sf9 cells infected with baculovirus using diMeK4H3-21 as substrate |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346862
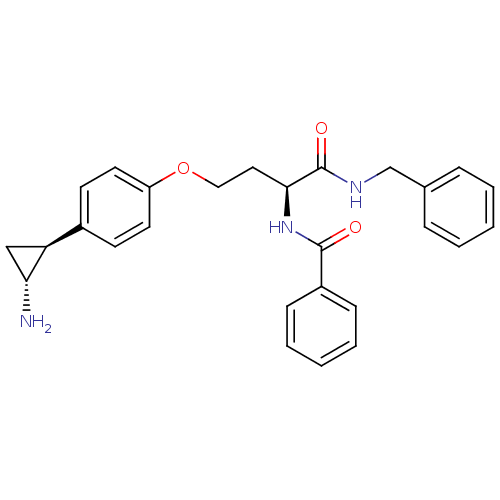
(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346534
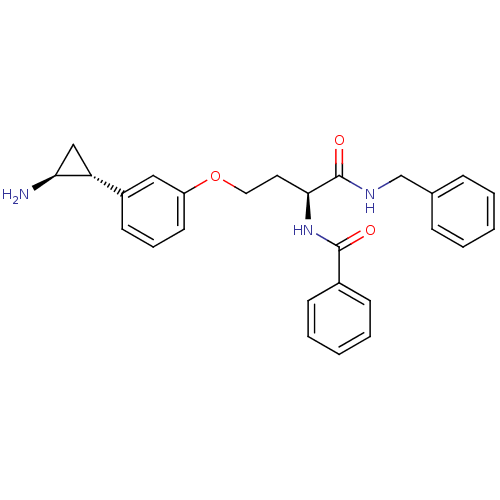
(CHEMBL1797705)Show SMILES N[C@H]1C[C@@H]1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346865
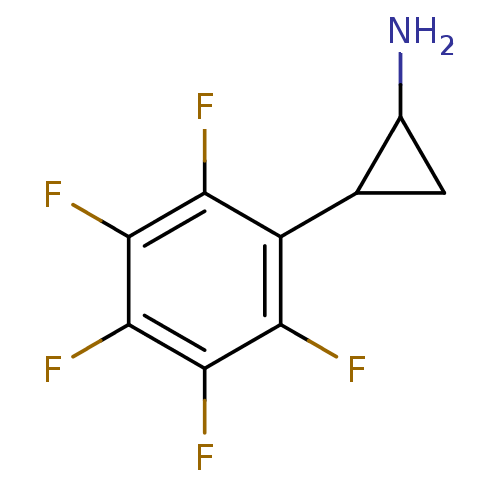
(2-PFPA | CHEMBL1797642)Show InChI InChI=1S/C9H6F5N/c10-5-4(2-1-3(2)15)6(11)8(13)9(14)7(5)12/h2-3H,1,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50113851

((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 catalytic domain |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396023

(CHEMBL2169919)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L using [3H]-SAM as substrate after 30 mins |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of GLP by fluorescence polarization assay in presence of fluorescein-labeled H3 peptide |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396027
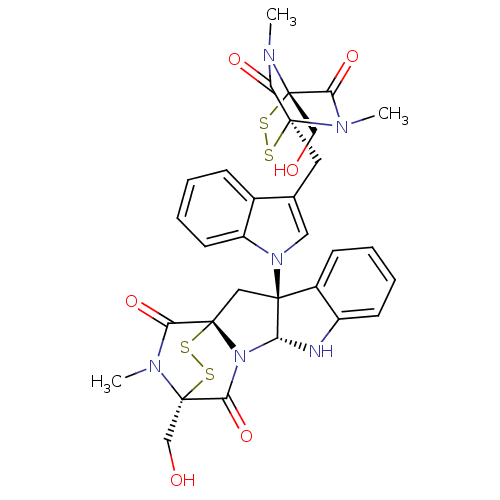
(CHEMBL499593)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)n1cc(C[C@]23SS[C@](CO)(N(C)C2=O)C(=O)N3C)c2ccccc12 |r,TLB:17:16:1.2:21.22,7:15:1.2:21.22| Show InChI InChI=1S/C31H30N6O6S4/c1-33-25(42)30(15-38)34(2)23(40)28(33,44-46-30)12-17-13-36(21-11-7-4-8-18(17)21)27-14-29-24(41)35(3)31(16-39,47-45-29)26(43)37(29)22(27)32-20-10-6-5-9-19(20)27/h4-11,13,22,32,38-39H,12,14-16H2,1-3H3/t22-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of G9a in human MCF7 cells after 48 hrs by clonogenic assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of G9a in human MDA-MB-231 cells after 48 hrs by clonogenic assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50396019
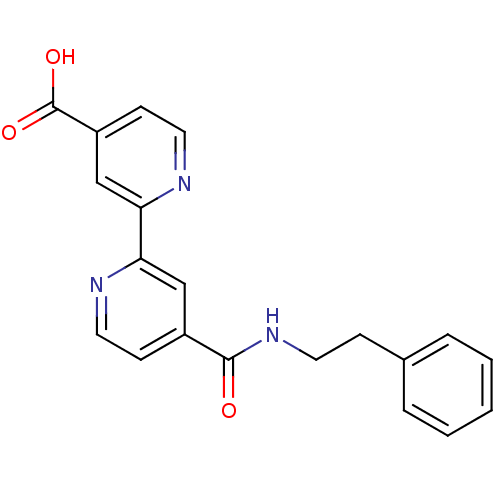
(4'-(phenethylcarbamoyl)-[2,2'-bipyridine]-...)Show SMILES OC(=O)c1ccnc(c1)-c1cc(ccn1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C20H17N3O3/c24-19(23-9-6-14-4-2-1-3-5-14)15-7-10-21-17(12-15)18-13-16(20(25)26)8-11-22-18/h1-5,7-8,10-13H,6,9H2,(H,23,24)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2E catalytic domain by mass spectrophotometric analysis |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396016
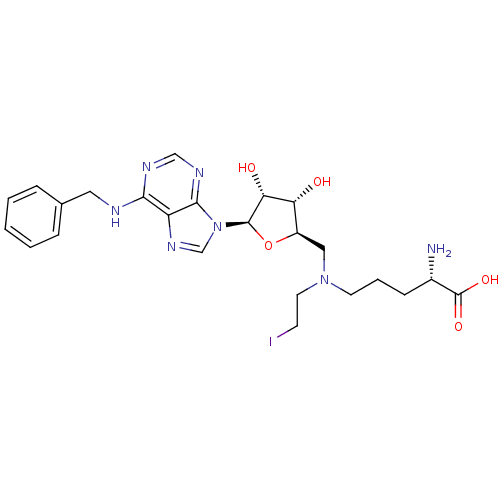
(CHEMBL2169918)Show SMILES N[C@@H](CCCN(CCI)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H32IN7O5/c25-8-10-31(9-4-7-16(26)24(35)36)12-17-19(33)20(34)23(37-17)32-14-30-18-21(28-13-29-22(18)32)27-11-15-5-2-1-3-6-15/h1-3,5-6,13-14,16-17,19-20,23,33-34H,4,7-12,26H2,(H,35,36)(H,27,28,29)/t16-,17+,19+,20+,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human DOT1L amino acid 1 to 472 expressed in Escherichia coli BL21 (DE3) using [3H]-SAM as substrate preincubated for 10 min... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396017
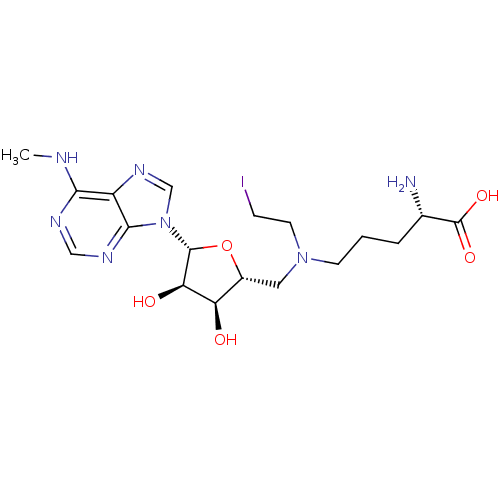
(CHEMBL2172427)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCI)CCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H28IN7O5/c1-21-15-12-16(23-8-22-15)26(9-24-12)17-14(28)13(27)11(31-17)7-25(6-4-19)5-2-3-10(20)18(29)30/h8-11,13-14,17,27-28H,2-7,20H2,1H3,(H,29,30)(H,21,22,23)/t10-,11+,13+,14+,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human DOT1L amino acid 1 to 472 expressed in Escherichia coli BL21 (DE3) using [3H]-SAM as substrate preincubated for 10 min... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50396022

(CHEMBL2169920)Show SMILES Oc1ccc(CCNCCN(C2CCCCC2)C(=O)CCNCCc2ccc(Cl)c(Cl)c2)c2OCC(=O)Nc12 Show InChI InChI=1S/C29H38Cl2N4O4/c30-23-8-6-20(18-24(23)31)10-13-32-15-12-27(38)35(22-4-2-1-3-5-22)17-16-33-14-11-21-7-9-25(36)28-29(21)39-19-26(37)34-28/h6-9,18,22,32-33,36H,1-5,10-17,19H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human SMYD2 amino acid 1 to 433 expressed in Escherichia coli BL21 (DE3) using Biotinaminohexanoyl- GSRAHSSHLKSKKGQSTSRH as... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396027

(CHEMBL499593)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)n1cc(C[C@]23SS[C@](CO)(N(C)C2=O)C(=O)N3C)c2ccccc12 |r,TLB:17:16:1.2:21.22,7:15:1.2:21.22| Show InChI InChI=1S/C31H30N6O6S4/c1-33-25(42)30(15-38)34(2)23(40)28(33,44-46-30)12-17-13-36(21-11-7-4-8-18(17)21)27-14-29-24(41)35(3)31(16-39,47-45-29)26(43)37(29)22(27)32-20-10-6-5-9-19(20)27/h4-11,13,22,32,38-39H,12,14-16H2,1-3H3/t22-,27+,28+,29+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of G9a |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50396020
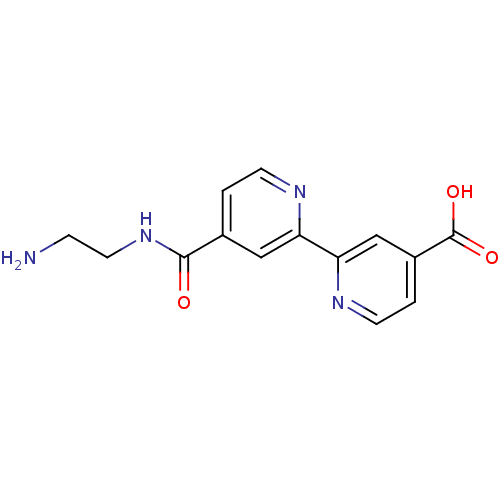
(CHEMBL1615036)Show InChI InChI=1S/C14H14N4O3/c15-3-6-18-13(19)9-1-4-16-11(7-9)12-8-10(14(20)21)2-5-17-12/h1-2,4-5,7-8H,3,6,15H2,(H,18,19)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2E catalytic domain by mass spectrophotometric analysis |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50134315
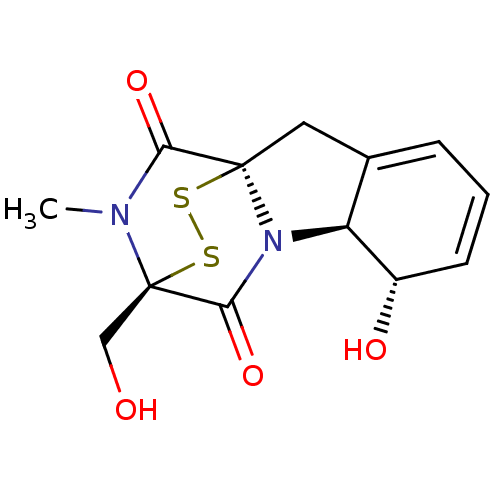
(7-hydroxy-11-hydroxymethyl-12-methyl-14,15-dithia-...)Show SMILES CN1C(=O)[C@]23CC4=CC=C[C@H](O)[C@H]4N2C(=O)[C@@]1(CO)SS3 |r,c:8,t:6,THB:12:13:1.2:19.20,15:14:1.2:19.20| Show InChI InChI=1S/C13H14N2O4S2/c1-14-10(18)12-5-7-3-2-4-8(17)9(7)15(12)11(19)13(14,6-16)21-20-12/h2-4,8-9,16-17H,5-6H2,1H3/t8-,9-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50185388
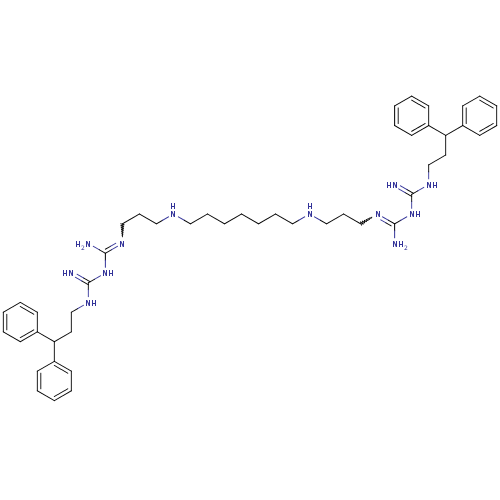
(CHEMBL378900 | N-(3,3-diphenylpropyl)-1-3-[3-({7-[...)Show SMILES NC(NC(=N)NCCC(c1ccccc1)c1ccccc1)=NCCCNCCCCCCCNCCCN=C(N)NC(=N)NCCC(c1ccccc1)c1ccccc1 |w:21.23,37.38| Show InChI InChI=1S/C47H68N12/c48-44(58-46(50)56-36-28-42(38-20-8-4-9-21-38)39-22-10-5-11-23-39)54-34-18-32-52-30-16-2-1-3-17-31-53-33-19-35-55-45(49)59-47(51)57-37-29-43(40-24-12-6-13-25-40)41-26-14-7-15-27-41/h4-15,20-27,42-43,52-53H,1-3,16-19,28-37H2,(H5,48,50,54,56,58)(H5,49,51,55,57,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6x His-tagged human LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate by luminold... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50396013
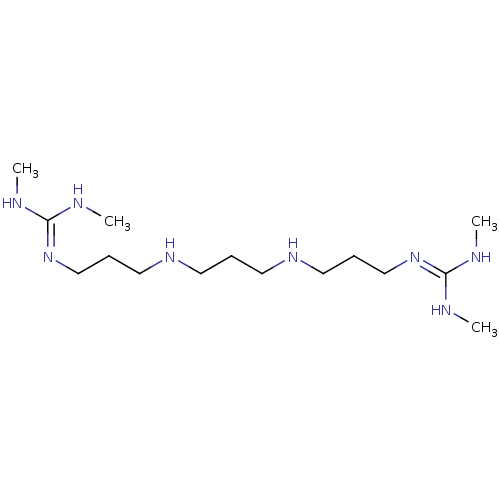
(CHEMBL1198896)Show SMILES [#6]-[#7]\[#6](-[#7]-[#6])=[#7]/[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7]-[#6])-[#7]-[#6] Show InChI InChI=1S/C15H36N8/c1-16-14(17-2)22-12-6-10-20-8-5-9-21-11-7-13-23-15(18-3)19-4/h20-21H,5-13H2,1-4H3,(H2,16,17,22)(H2,18,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6x His-tagged human LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate by luminold... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361470
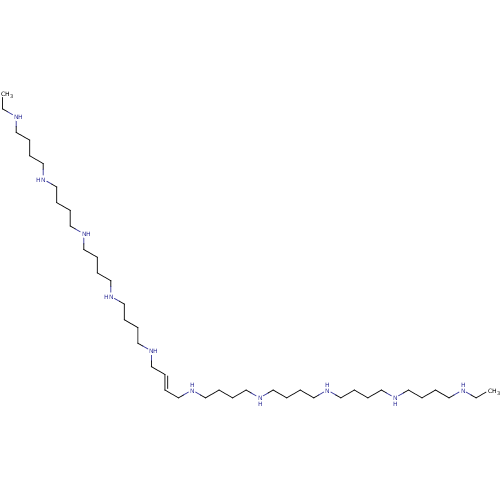
(CHEMBL226102)Show SMILES CCNCCCCNCCCCNCCCCNCCCCNC\C=C\CNCCCCNCCCCNCCCCNCCCCNCC Show InChI InChI=1S/C40H90N10/c1-3-41-23-5-7-25-43-27-9-11-29-45-31-13-15-33-47-35-17-19-37-49-39-21-22-40-50-38-20-18-36-48-34-16-14-32-46-30-12-10-28-44-26-8-6-24-42-4-2/h21-22,41-50H,3-20,23-40H2,1-2H3/b22-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6x His-tagged human LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate by luminold... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50396021

(CHEMBL1196431)Show SMILES CCNCCCCNCCCCNCCCCNCCCCNC\C=C/CNCCCCNCCCCNCCCCNCCCCNCC Show InChI InChI=1S/C40H90N10/c1-3-41-23-5-7-25-43-27-9-11-29-45-31-13-15-33-47-35-17-19-37-49-39-21-22-40-50-38-20-18-36-48-34-16-14-32-46-30-12-10-28-44-26-8-6-24-42-4-2/h21-22,41-50H,3-20,23-40H2,1-2H3/b22-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal 6x His-tagged human LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate by luminold... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396026
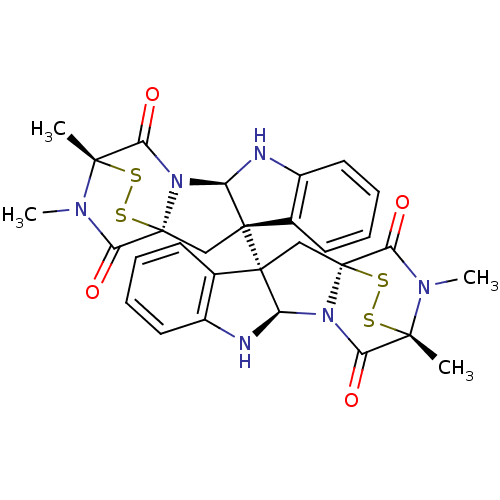
(CHEMBL2172426)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(C)SS3)[C@]12C[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,TLB:17:16:1.2:20.21,7:15:1.2:20.21,THB:34:33:29.31:26.25,36:35:29.31:26.25| Show InChI InChI=1S/C30H28N6O4S4/c1-25-21(37)35-19-27(15-9-5-7-11-17(15)31-19,13-29(35,43-41-25)23(39)33(25)3)28-14-30-24(40)34(4)26(2,42-44-30)22(38)36(30)20(28)32-18-12-8-6-10-16(18)28/h5-12,19-20,31-32H,13-14H2,1-4H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50134315

(7-hydroxy-11-hydroxymethyl-12-methyl-14,15-dithia-...)Show SMILES CN1C(=O)[C@]23CC4=CC=C[C@H](O)[C@H]4N2C(=O)[C@@]1(CO)SS3 |r,c:8,t:6,THB:12:13:1.2:19.20,15:14:1.2:19.20| Show InChI InChI=1S/C13H14N2O4S2/c1-14-10(18)12-5-7-3-2-4-8(17)9(7)15(12)11(19)13(14,6-16)21-20-12/h2-4,8-9,16-17H,5-6H2,1H3/t8-,9-,12+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of G9a |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase Su(var)3-9
(Drosophila melanogaster) | BDBM50315537
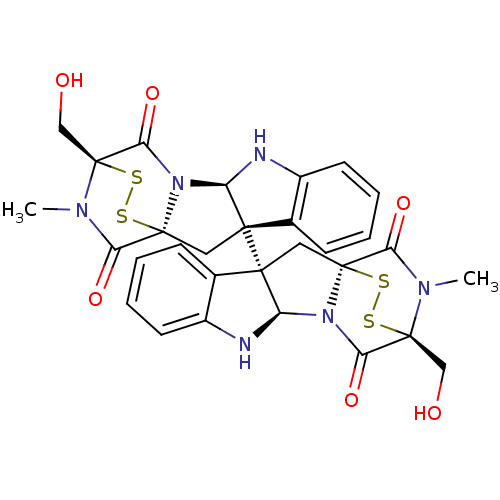
(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Drosophila melanogaster SUV39 by radioactive filter-binding assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396026

(CHEMBL2172426)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(C)SS3)[C@]12C[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,TLB:17:16:1.2:20.21,7:15:1.2:20.21,THB:34:33:29.31:26.25,36:35:29.31:26.25| Show InChI InChI=1S/C30H28N6O4S4/c1-25-21(37)35-19-27(15-9-5-7-11-17(15)31-19,13-29(35,43-41-25)23(39)33(25)3)28-14-30-24(40)34(4)26(2,42-44-30)22(38)36(30)20(28)32-18-12-8-6-10-16(18)28/h5-12,19-20,31-32H,13-14H2,1-4H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of G9a |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50300028
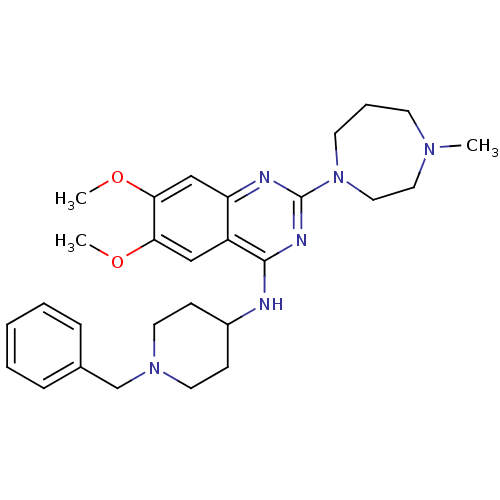
(CHEMBL569864 | N-(1-benzylpiperidin-4-yl)-6,7-dime...)Show SMILES COc1cc2nc(nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC)N1CCCN(C)CC1 Show InChI InChI=1S/C28H38N6O2/c1-32-12-7-13-34(17-16-32)28-30-24-19-26(36-3)25(35-2)18-23(24)27(31-28)29-22-10-14-33(15-11-22)20-21-8-5-4-6-9-21/h4-6,8-9,18-19,22H,7,10-17,20H2,1-3H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged GLP SET domain amino acid 951 to 1235 expressed in Escherichia coli BL21 (DE3) using Histone H3 p... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50315537

(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50396014

(CHEMBL2169888)Show SMILES N[C@@H]1C[C@H]1c1cc(F)cc(F)c1OCc1ccccc1 |r| Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396024
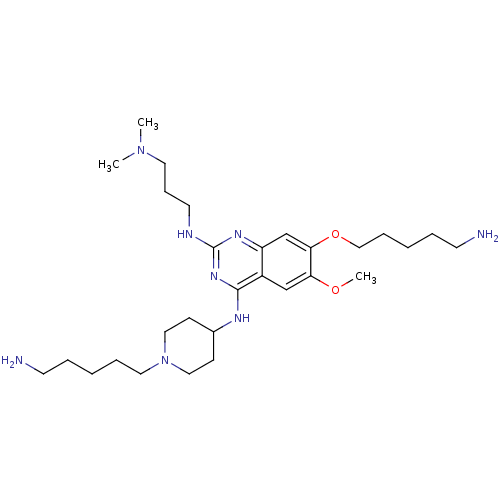
(CHEMBL1232453)Show SMILES COc1cc2c(NC3CCN(CCCCCN)CC3)nc(NCCCN(C)C)nc2cc1OCCCCCN Show InChI InChI=1S/C29H52N8O2/c1-36(2)16-10-15-32-29-34-25-22-27(39-20-9-5-7-14-31)26(38-3)21-24(25)28(35-29)33-23-11-18-37(19-12-23)17-8-4-6-13-30/h21-23H,4-20,30-31H2,1-3H3,(H2,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant G9a catalytic domain amino acid 913 to 1193 expressed in Escherichia coli BL21 (DE3) by mass spectrophotometric assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396029
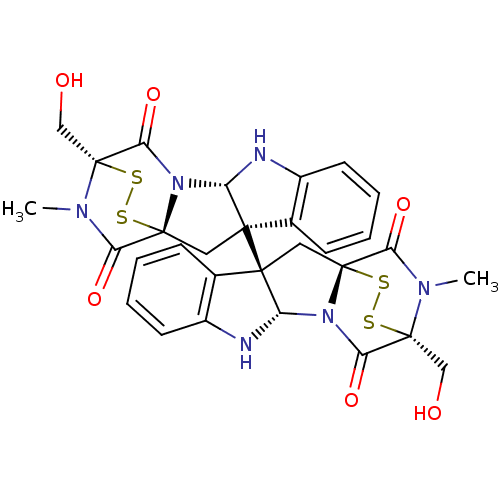
(CHEMBL1222849)Show SMILES CN1C(=O)[C@]23C[C@@]4([C@@H](Nc5ccccc45)N2C(=O)[C@@]1(CO)SS3)[C@@]12C[C@@]34SS[C@@](CO)(N(C)C3=O)C(=O)N4[C@@H]1Nc1ccccc21 |r,TLB:38:37:31.33:27.26,36:35:31.33:27.26,THB:17:16:1.2:21.22,7:15:1.2:21.22| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged G9a after 1 hr by radioactive filter-binding assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50396018
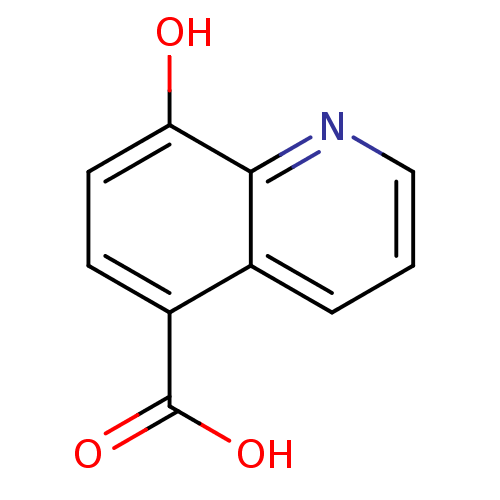
(CHEMBL1230640)Show InChI InChI=1S/C10H7NO3/c12-8-4-3-7(10(13)14)6-2-1-5-11-9(6)8/h1-5,12H,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2A catalytic domain by mass spectrophotometric analysis |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300028

(CHEMBL569864 | N-(1-benzylpiperidin-4-yl)-6,7-dime...)Show SMILES COc1cc2nc(nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC)N1CCCN(C)CC1 Show InChI InChI=1S/C28H38N6O2/c1-32-12-7-13-34(17-16-32)28-30-24-19-26(36-3)25(35-2)18-23(24)27(31-28)29-22-10-14-33(15-11-22)20-21-8-5-4-6-9-21/h4-6,8-9,18-19,22H,7,10-17,20H2,1-3H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged G9a SET domain amino acid 913 to 1193 expressed in Escherichia coli BL21 (DE3) using Histone H3 p... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346862

(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50315537

(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged G9a after 1 hr by radioactive filter-binding assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50396018

(CHEMBL1230640)Show InChI InChI=1S/C10H7NO3/c12-8-4-3-7(10(13)14)6-2-1-5-11-9(6)8/h1-5,12H,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2E catalytic domain by mass spectrophotometric analysis |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50361472
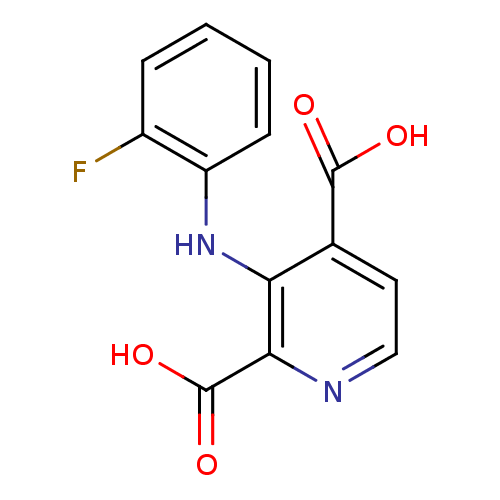
(CHEMBL1938896)Show InChI InChI=1S/C13H9FN2O4/c14-8-3-1-2-4-9(8)16-10-7(12(17)18)5-6-15-11(10)13(19)20/h1-6,16H,(H,17,18)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human JMJD2E |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Mus musculus) | BDBM50315537

(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse G9a |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346534

(CHEMBL1797705)Show SMILES N[C@H]1C[C@@H]1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N terminal hexahistidine-tag recombinant human LSD1 expressed in Escherichia coli BL21 (DE3) using histone H3 peptide as substrate prei... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4C
(Homo sapiens (Human)) | BDBM50361475
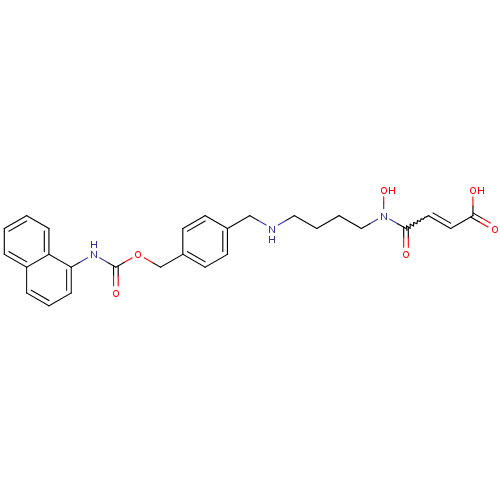
(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-fused human JMJD2C catalytic domain amino acid 1 to 420 using H3K9me3 as substrate by mass spectrophotometric analysis |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2A |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50361475

(CHEMBL1938900)Show SMILES ON(CCCCNCc1ccc(COC(=O)Nc2cccc3ccccc23)cc1)C(=O)C=CC(O)=O |w:31.33| Show InChI InChI=1S/C27H29N3O6/c31-25(14-15-26(32)33)30(35)17-4-3-16-28-18-20-10-12-21(13-11-20)19-36-27(34)29-24-9-5-7-22-6-1-2-8-23(22)24/h1-2,5-15,28,35H,3-4,16-19H2,(H,29,34)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JMJD2E |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396025

(CHEMBL1089088)Show SMILES CS[C@@]12CC3=CC=C[C@H](OC(C)=O)[C@H]3N1C(=O)[C@@](CO)(SC)N(C)C2=O |r,c:6,t:4| Show InChI InChI=1S/C17H22N2O5S2/c1-10(21)24-12-7-5-6-11-8-16(25-3)14(22)18(2)17(9-20,26-4)15(23)19(16)13(11)12/h5-7,12-13,20H,8-9H2,1-4H3/t12-,13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396025

(CHEMBL1089088)Show SMILES CS[C@@]12CC3=CC=C[C@H](OC(C)=O)[C@H]3N1C(=O)[C@@](CO)(SC)N(C)C2=O |r,c:6,t:4| Show InChI InChI=1S/C17H22N2O5S2/c1-10(21)24-12-7-5-6-11-8-16(25-3)14(22)18(2)17(9-20,26-4)15(23)19(16)13(11)12/h5-7,12-13,20H,8-9H2,1-4H3/t12-,13-,16+,17+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of G9a |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396028
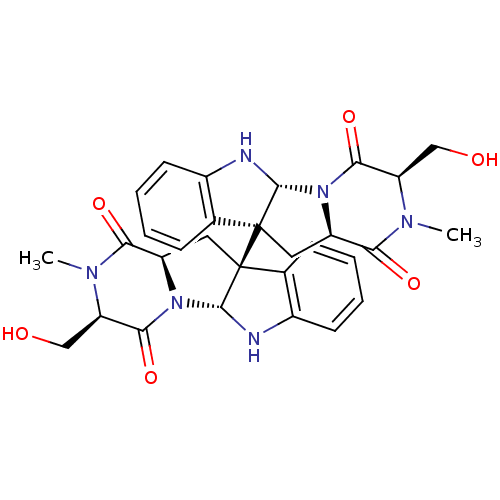
(CHEMBL1222848)Show SMILES CN1[C@H](CO)C(=O)N2[C@H](C[C@]3([C@@H]2Nc2ccccc32)[C@]23C[C@H]4N([C@H]2Nc2ccccc32)C(=O)[C@@H](CO)N(C)C4=O)C1=O |r| Show InChI InChI=1S/C30H32N6O6/c1-33-21(13-37)25(41)35-19(23(33)39)11-29(15-7-3-5-9-17(15)31-27(29)35)30-12-20-24(40)34(2)22(14-38)26(42)36(20)28(30)32-18-10-6-4-8-16(18)30/h3-10,19-22,27-28,31-32,37-38H,11-14H2,1-2H3/t19-,20-,21-,22-,27-,28-,29+,30+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged G9a after 1 hr by radioactive filter-binding assay |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data