 Found 85 hits Enz. Inhib. hit(s) with all data for entry = 50040611
Found 85 hits Enz. Inhib. hit(s) with all data for entry = 50040611 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980
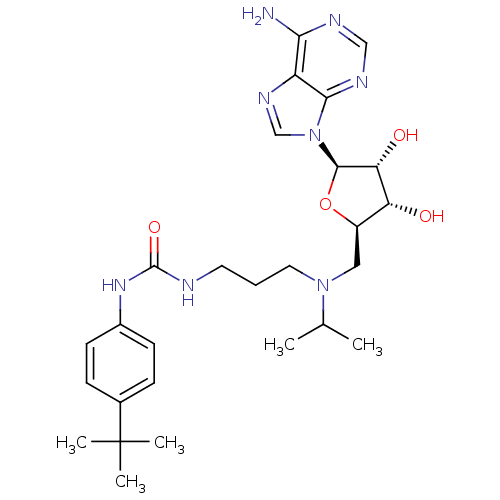
(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396979
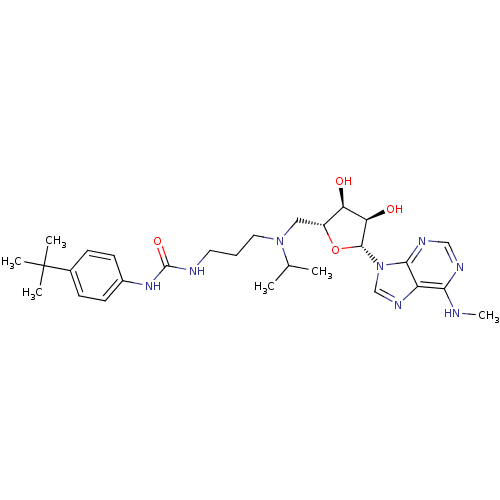
(CHEMBL2171170)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCCNC(=O)Nc2ccc(cc2)C(C)(C)C)C(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H42N8O4/c1-17(2)35(13-7-12-30-27(39)34-19-10-8-18(9-11-19)28(3,4)5)14-20-22(37)23(38)26(40-20)36-16-33-21-24(29-6)31-15-32-25(21)36/h8-11,15-17,20,22-23,26,37-38H,7,12-14H2,1-6H3,(H,29,31,32)(H2,30,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396978
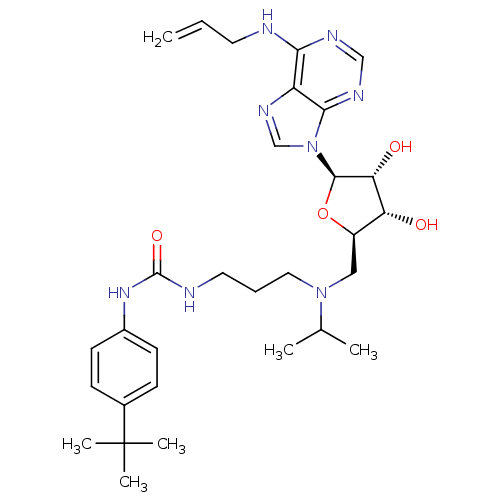
(CHEMBL2171171)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC=C)ncnc12 |r| Show InChI InChI=1S/C30H44N8O4/c1-7-13-31-26-23-27(34-17-33-26)38(18-35-23)28-25(40)24(39)22(42-28)16-37(19(2)3)15-8-14-32-29(41)36-21-11-9-20(10-12-21)30(4,5)6/h7,9-12,17-19,22,24-25,28,39-40H,1,8,13-16H2,2-6H3,(H,31,33,34)(H2,32,36,41)/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396977
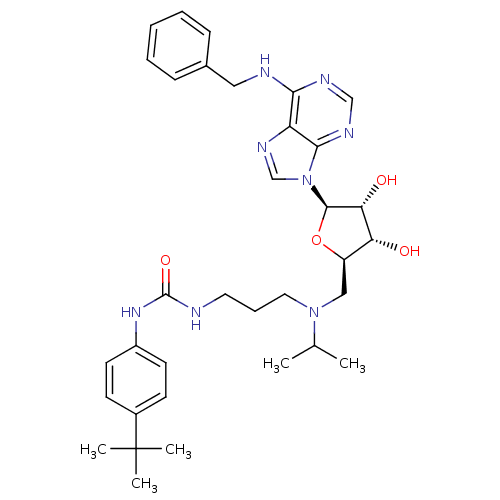
(CHEMBL2171172)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C34H46N8O4/c1-22(2)41(17-9-16-35-33(45)40-25-14-12-24(13-15-25)34(3,4)5)19-26-28(43)29(44)32(46-26)42-21-39-27-30(37-20-38-31(27)42)36-18-23-10-7-6-8-11-23/h6-8,10-15,20-22,26,28-29,32,43-44H,9,16-19H2,1-5H3,(H2,35,40,45)(H,36,37,38)/t26-,28-,29-,32-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396988
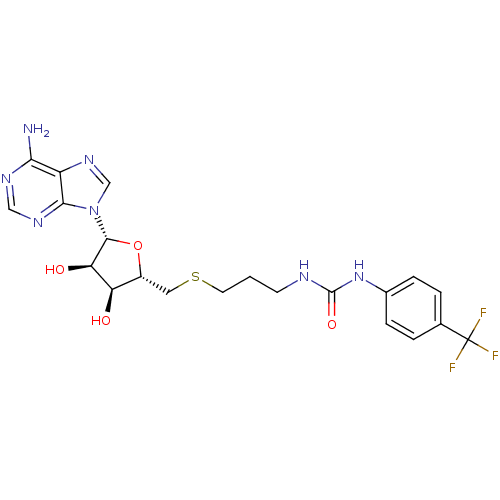
(CHEMBL2170997)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(cc2)C(F)(F)F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24F3N7O4S/c22-21(23,24)11-2-4-12(5-3-11)30-20(34)26-6-1-7-36-8-13-15(32)16(33)19(35-13)31-10-29-14-17(25)27-9-28-18(14)31/h2-5,9-10,13,15-16,19,32-33H,1,6-8H2,(H2,25,27,28)(H2,26,30,34)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396992
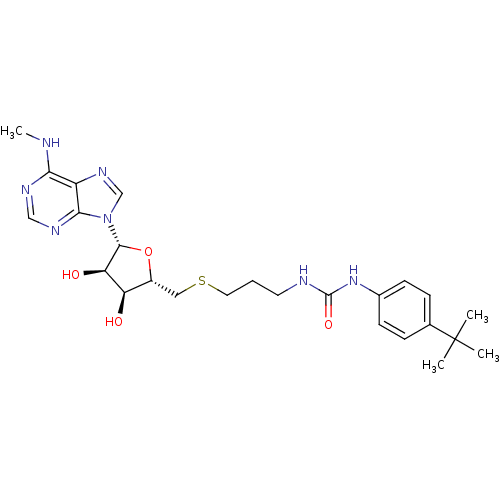
(CHEMBL2170993)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(cc2)C(C)(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C25H35N7O4S/c1-25(2,3)15-6-8-16(9-7-15)31-24(35)27-10-5-11-37-12-17-19(33)20(34)23(36-17)32-14-30-18-21(26-4)28-13-29-22(18)32/h6-9,13-14,17,19-20,23,33-34H,5,10-12H2,1-4H3,(H,26,28,29)(H2,27,31,35)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396983
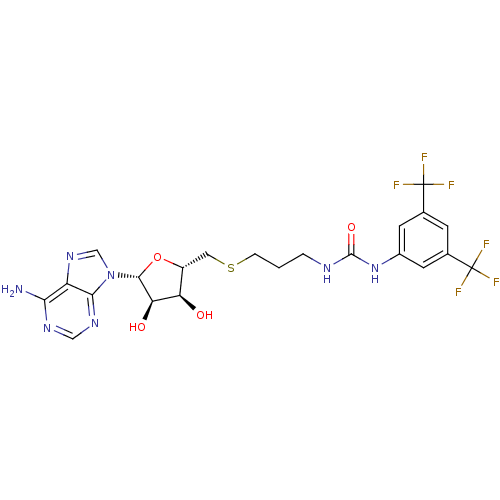
(CHEMBL2171002)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H23F6N7O4S/c23-21(24,25)10-4-11(22(26,27)28)6-12(5-10)34-20(38)30-2-1-3-40-7-13-15(36)16(37)19(39-13)35-9-33-14-17(29)31-8-32-18(14)35/h4-6,8-9,13,15-16,19,36-37H,1-3,7H2,(H2,29,31,32)(H2,30,34,38)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396991
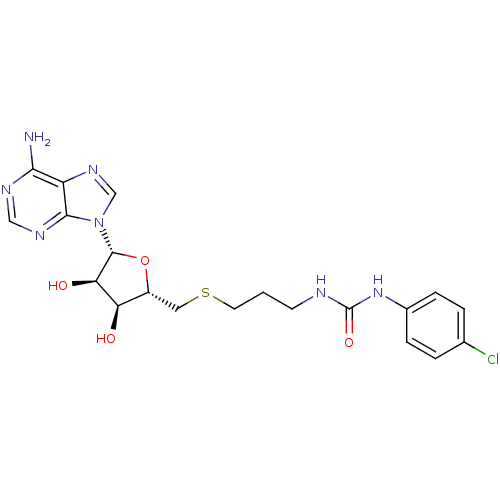
(CHEMBL2170994)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(Cl)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24ClN7O4S/c21-11-2-4-12(5-3-11)27-20(31)23-6-1-7-33-8-13-15(29)16(30)19(32-13)28-10-26-14-17(22)24-9-25-18(14)28/h2-5,9-10,13,15-16,19,29-30H,1,6-8H2,(H2,22,24,25)(H2,23,27,31)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396985
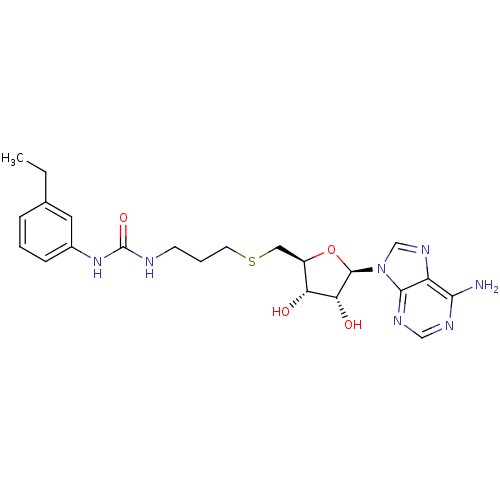
(CHEMBL2171000)Show SMILES CCc1cccc(NC(=O)NCCCSC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)c1 |r| Show InChI InChI=1S/C22H29N7O4S/c1-2-13-5-3-6-14(9-13)28-22(32)24-7-4-8-34-10-15-17(30)18(31)21(33-15)29-12-27-16-19(23)25-11-26-20(16)29/h3,5-6,9,11-12,15,17-18,21,30-31H,2,4,7-8,10H2,1H3,(H2,23,25,26)(H2,24,28,32)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396987
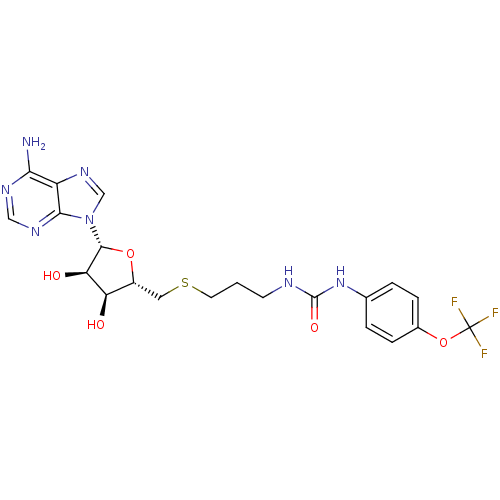
(CHEMBL2170998)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(OC(F)(F)F)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24F3N7O5S/c22-21(23,24)36-12-4-2-11(3-5-12)30-20(34)26-6-1-7-37-8-13-15(32)16(33)19(35-13)31-10-29-14-17(25)27-9-28-18(14)31/h2-5,9-10,13,15-16,19,32-33H,1,6-8H2,(H2,25,27,28)(H2,26,30,34)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396990
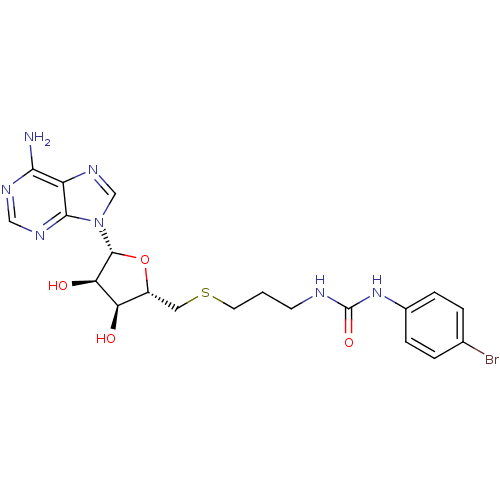
(CHEMBL2170995)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(Br)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24BrN7O4S/c21-11-2-4-12(5-3-11)27-20(31)23-6-1-7-33-8-13-15(29)16(30)19(32-13)28-10-26-14-17(22)24-9-25-18(14)28/h2-5,9-10,13,15-16,19,29-30H,1,6-8H2,(H2,22,24,25)(H2,23,27,31)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396989
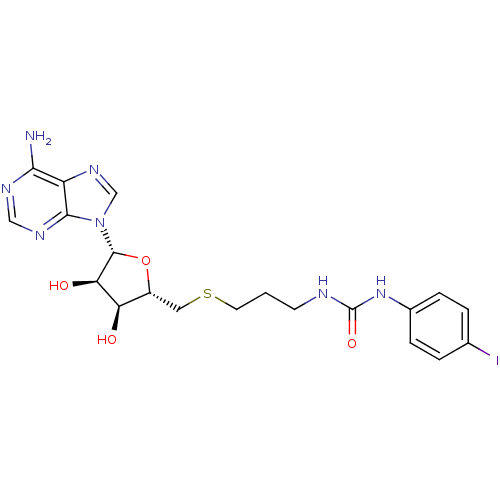
(CHEMBL2170996)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(I)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24IN7O4S/c21-11-2-4-12(5-3-11)27-20(31)23-6-1-7-33-8-13-15(29)16(30)19(32-13)28-10-26-14-17(22)24-9-25-18(14)28/h2-5,9-10,13,15-16,19,29-30H,1,6-8H2,(H2,22,24,25)(H2,23,27,31)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396981
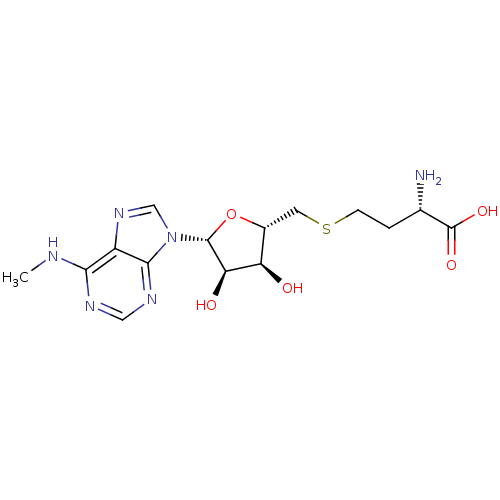
(CHEMBL2171174)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H22N6O5S/c1-17-12-9-13(19-5-18-12)21(6-20-9)14-11(23)10(22)8(26-14)4-27-3-2-7(16)15(24)25/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H,24,25)(H,17,18,19)/t7-,8+,10+,11+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396993
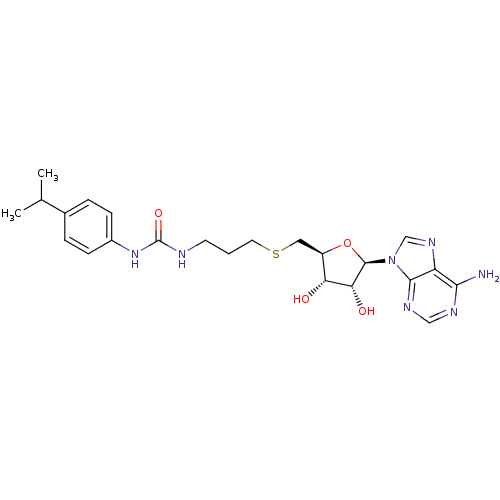
(CHEMBL2170992)Show SMILES CC(C)c1ccc(NC(=O)NCCCSC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)cc1 |r| Show InChI InChI=1S/C23H31N7O4S/c1-13(2)14-4-6-15(7-5-14)29-23(33)25-8-3-9-35-10-16-18(31)19(32)22(34-16)30-12-28-17-20(24)26-11-27-21(17)30/h4-7,11-13,16,18-19,22,31-32H,3,8-10H2,1-2H3,(H2,24,26,27)(H2,25,29,33)/t16-,18-,19-,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PRMT1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396996
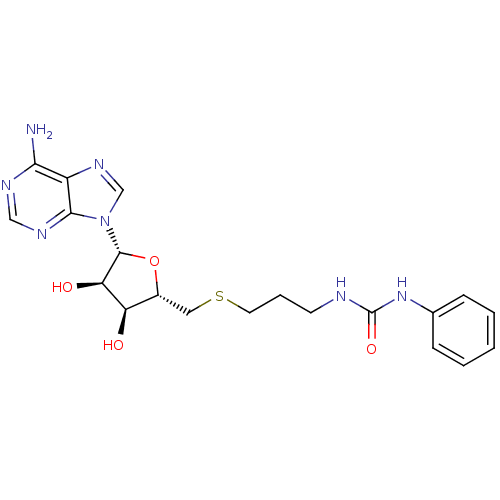
(CHEMBL2170989)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H25N7O4S/c21-17-14-18(24-10-23-17)27(11-25-14)19-16(29)15(28)13(31-19)9-32-8-4-7-22-20(30)26-12-5-2-1-3-6-12/h1-3,5-6,10-11,13,15-16,19,28-29H,4,7-9H2,(H2,21,23,24)(H2,22,26,30)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396986
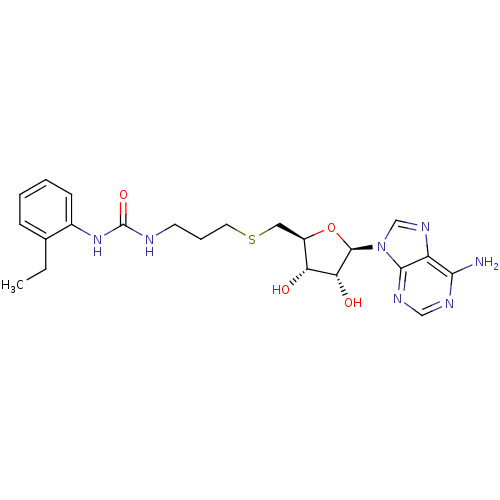
(CHEMBL2170999)Show SMILES CCc1ccccc1NC(=O)NCCCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H29N7O4S/c1-2-13-6-3-4-7-14(13)28-22(32)24-8-5-9-34-10-15-17(30)18(31)21(33-15)29-12-27-16-19(23)25-11-26-20(16)29/h3-4,6-7,11-12,15,17-18,21,30-31H,2,5,8-10H2,1H3,(H2,23,25,26)(H2,24,28,32)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of CARM1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396984
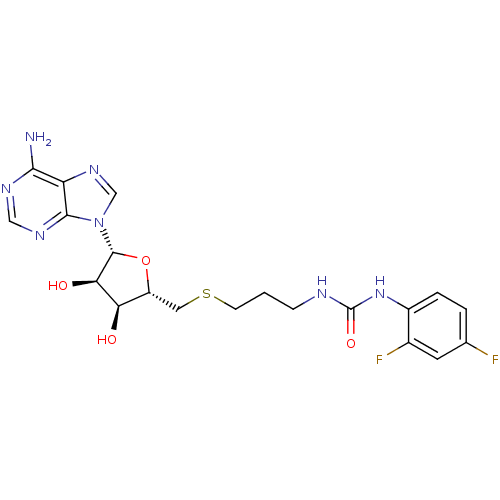
(CHEMBL2171001)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(F)cc2F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H23F2N7O4S/c21-10-2-3-12(11(22)6-10)28-20(32)24-4-1-5-34-7-13-15(30)16(31)19(33-13)29-9-27-14-17(23)25-8-26-18(14)29/h2-3,6,8-9,13,15-16,19,30-31H,1,4-5,7H2,(H2,23,25,26)(H2,24,28,32)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396994
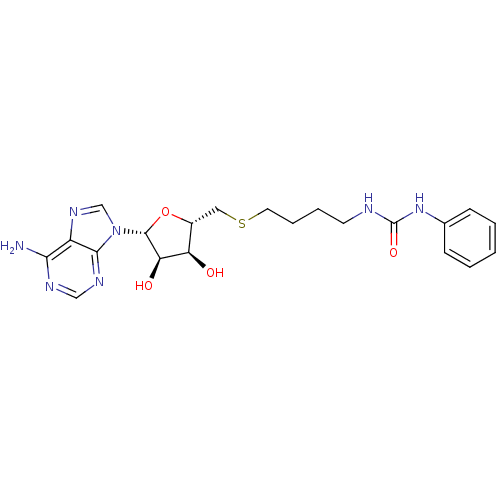
(CHEMBL2170991)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCCNC(=O)Nc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H27N7O4S/c22-18-15-19(25-11-24-18)28(12-26-15)20-17(30)16(29)14(32-20)10-33-9-5-4-8-23-21(31)27-13-6-2-1-3-7-13/h1-3,6-7,11-12,14,16-17,20,29-30H,4-5,8-10H2,(H2,22,24,25)(H2,23,27,31)/t14-,16-,17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397022
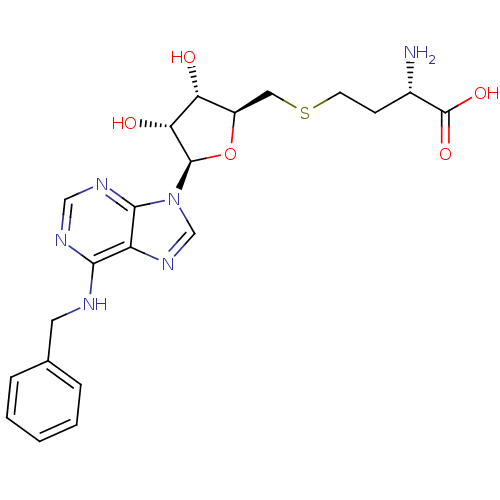
(CHEMBL2171182)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3)ncnc12)C(O)=O |r| Show InChI InChI=1S/C21H26N6O5S/c22-13(21(30)31)6-7-33-9-14-16(28)17(29)20(32-14)27-11-26-15-18(24-10-25-19(15)27)23-8-12-4-2-1-3-5-12/h1-5,10-11,13-14,16-17,20,28-29H,6-9,22H2,(H,30,31)(H,23,24,25)/t13-,14+,16+,17+,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397000
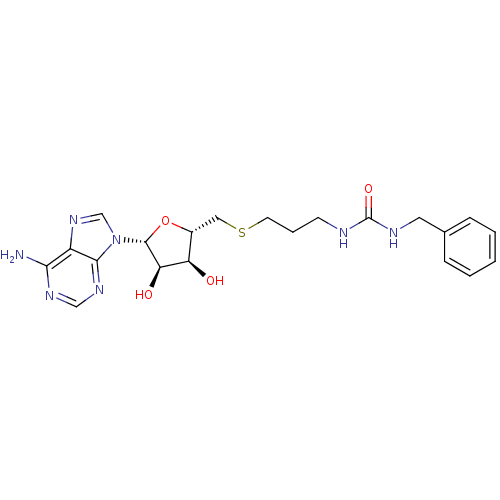
(CHEMBL2170985)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)NCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H27N7O4S/c22-18-15-19(26-11-25-18)28(12-27-15)20-17(30)16(29)14(32-20)10-33-8-4-7-23-21(31)24-9-13-5-2-1-3-6-13/h1-3,5-6,11-12,14,16-17,20,29-30H,4,7-10H2,(H2,22,25,26)(H2,23,24,31)/t14-,16-,17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397028
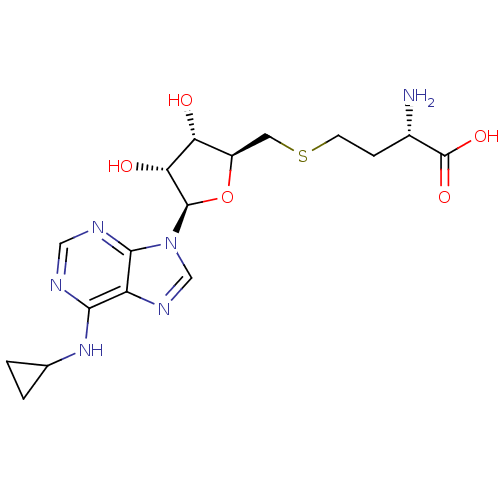
(CHEMBL2171176)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CC3)ncnc12)C(O)=O |r| Show InChI InChI=1S/C17H24N6O5S/c18-9(17(26)27)3-4-29-5-10-12(24)13(25)16(28-10)23-7-21-11-14(22-8-1-2-8)19-6-20-15(11)23/h6-10,12-13,16,24-25H,1-5,18H2,(H,26,27)(H,19,20,22)/t9-,10+,12+,13+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397029
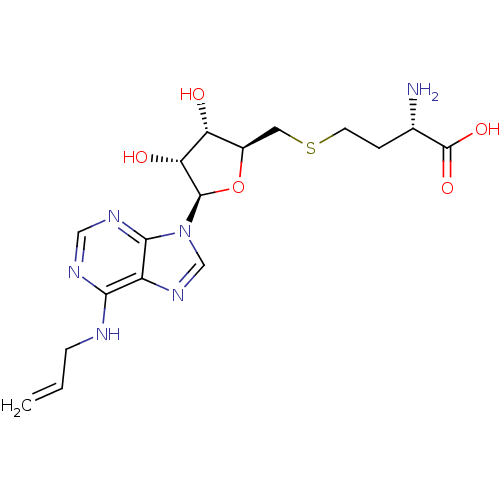
(CHEMBL2171175)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC=C)ncnc12)C(O)=O |r| Show InChI InChI=1S/C17H24N6O5S/c1-2-4-19-14-11-15(21-7-20-14)23(8-22-11)16-13(25)12(24)10(28-16)6-29-5-3-9(18)17(26)27/h2,7-10,12-13,16,24-25H,1,3-6,18H2,(H,26,27)(H,19,20,21)/t9-,10+,12+,13+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396982
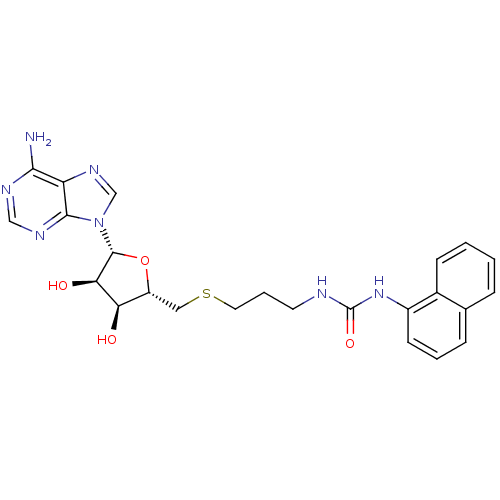
(CHEMBL2171168)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2cccc3ccccc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H27N7O4S/c25-21-18-22(28-12-27-21)31(13-29-18)23-20(33)19(32)17(35-23)11-36-10-4-9-26-24(34)30-16-8-3-6-14-5-1-2-7-15(14)16/h1-3,5-8,12-13,17,19-20,23,32-33H,4,9-11H2,(H2,25,27,28)(H2,26,30,34)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397026

(CHEMBL2171178)Show SMILES CCCCNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H28N6O5S/c1-2-3-5-20-15-12-16(22-8-21-15)24(9-23-12)17-14(26)13(25)11(29-17)7-30-6-4-10(19)18(27)28/h8-11,13-14,17,25-26H,2-7,19H2,1H3,(H,27,28)(H,20,21,22)/t10-,11+,13+,14+,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397027

(CHEMBL2171177)Show SMILES CC(C)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H26N6O5S/c1-8(2)22-14-11-15(20-6-19-14)23(7-21-11)16-13(25)12(24)10(28-16)5-29-4-3-9(18)17(26)27/h6-10,12-13,16,24-25H,3-5,18H2,1-2H3,(H,26,27)(H,19,20,22)/t9-,10+,12+,13+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396997
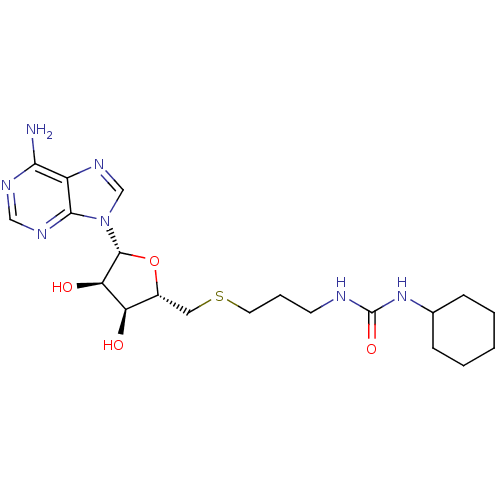
(CHEMBL2170988)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)NC2CCCCC2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H31N7O4S/c21-17-14-18(24-10-23-17)27(11-25-14)19-16(29)15(28)13(31-19)9-32-8-4-7-22-20(30)26-12-5-2-1-3-6-12/h10-13,15-16,19,28-29H,1-9H2,(H2,21,23,24)(H2,22,26,30)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396999
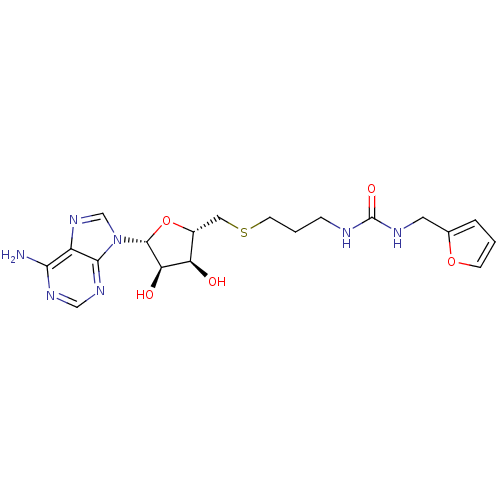
(CHEMBL2170986)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)NCc2ccco2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H25N7O5S/c20-16-13-17(24-9-23-16)26(10-25-13)18-15(28)14(27)12(31-18)8-32-6-2-4-21-19(29)22-7-11-3-1-5-30-11/h1,3,5,9-10,12,14-15,18,27-28H,2,4,6-8H2,(H2,20,23,24)(H2,21,22,29)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397024
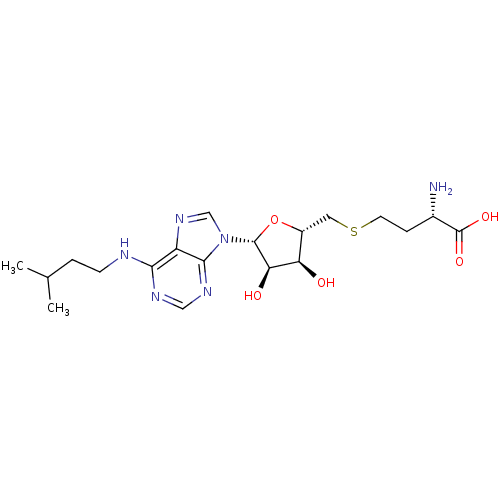
(CHEMBL2171180)Show SMILES CC(C)CCNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H30N6O5S/c1-10(2)3-5-21-16-13-17(23-8-22-16)25(9-24-13)18-15(27)14(26)12(30-18)7-31-6-4-11(20)19(28)29/h8-12,14-15,18,26-27H,3-7,20H2,1-2H3,(H,28,29)(H,21,22,23)/t11-,12+,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397025
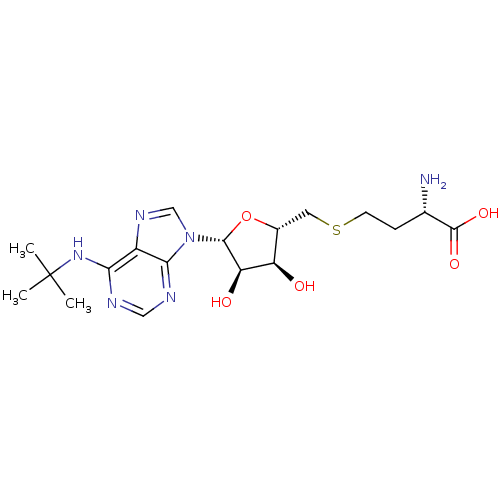
(CHEMBL2171179)Show SMILES CC(C)(C)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H28N6O5S/c1-18(2,3)23-14-11-15(21-7-20-14)24(8-22-11)16-13(26)12(25)10(29-16)6-30-5-4-9(19)17(27)28/h7-10,12-13,16,25-26H,4-6,19H2,1-3H3,(H,27,28)(H,20,21,23)/t9-,10+,12+,13+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397023
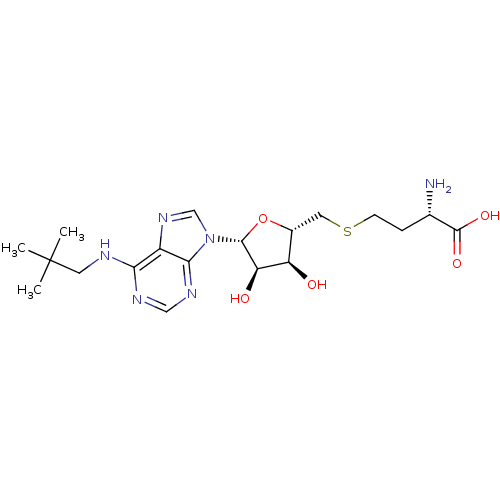
(CHEMBL2171181)Show SMILES CC(C)(C)CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H30N6O5S/c1-19(2,3)7-21-15-12-16(23-8-22-15)25(9-24-12)17-14(27)13(26)11(30-17)6-31-5-4-10(20)18(28)29/h8-11,13-14,17,26-27H,4-7,20H2,1-3H3,(H,28,29)(H,21,22,23)/t10-,11+,13+,14+,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397001
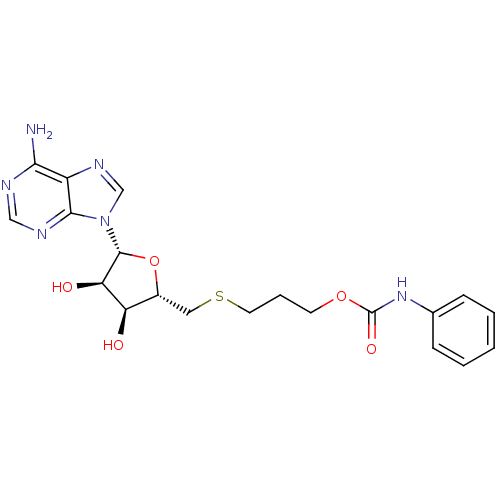
(CHEMBL2170984)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCOC(=O)Nc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24N6O5S/c21-17-14-18(23-10-22-17)26(11-24-14)19-16(28)15(27)13(31-19)9-32-8-4-7-30-20(29)25-12-5-2-1-3-6-12/h1-3,5-6,10-11,13,15-16,19,27-28H,4,7-9H2,(H,25,29)(H2,21,22,23)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396998
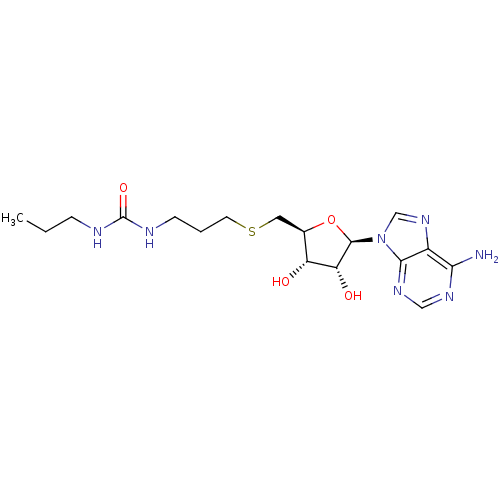
(CHEMBL2170987)Show SMILES CCCNC(=O)NCCCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C17H27N7O4S/c1-2-4-19-17(27)20-5-3-6-29-7-10-12(25)13(26)16(28-10)24-9-23-11-14(18)21-8-22-15(11)24/h8-10,12-13,16,25-26H,2-7H2,1H3,(H2,18,21,22)(H2,19,20,27)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396995
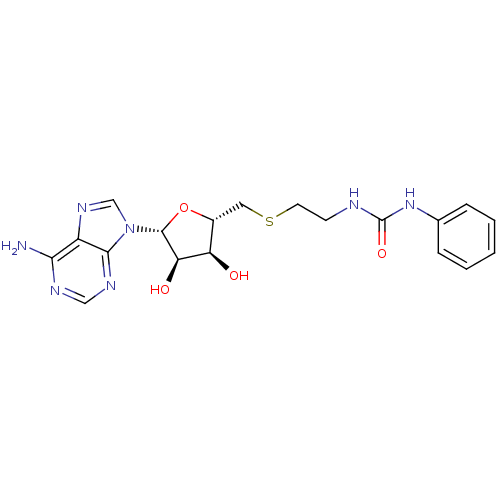
(CHEMBL2170990)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCNC(=O)Nc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H23N7O4S/c20-16-13-17(23-9-22-16)26(10-24-13)18-15(28)14(27)12(30-18)8-31-7-6-21-19(29)25-11-4-2-1-3-5-11/h1-5,9-10,12,14-15,18,27-28H,6-8H2,(H2,20,22,23)(H2,21,25,29)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397002
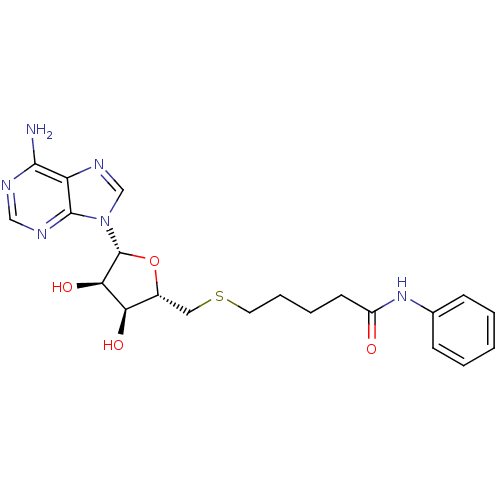
(CHEMBL2170983)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCCC(=O)Nc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H26N6O4S/c22-19-16-20(24-11-23-19)27(12-25-16)21-18(30)17(29)14(31-21)10-32-9-5-4-8-15(28)26-13-6-2-1-3-7-13/h1-3,6-7,11-12,14,17-18,21,29-30H,4-5,8-10H2,(H,26,28)(H2,22,23,24)/t14-,17-,18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397006
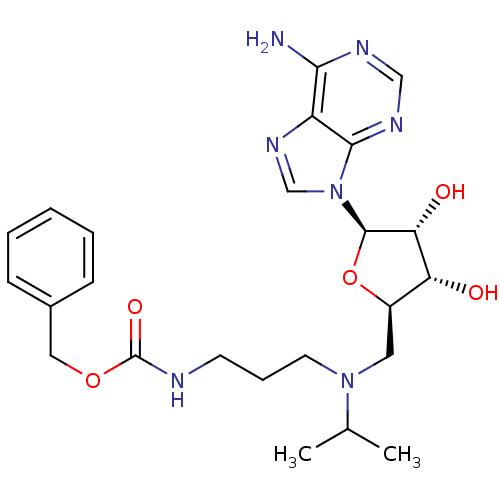
(CHEMBL2170979)Show SMILES CC(C)N(CCCNC(=O)OCc1ccccc1)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C24H33N7O5/c1-15(2)30(10-6-9-26-24(34)35-12-16-7-4-3-5-8-16)11-17-19(32)20(33)23(36-17)31-14-29-18-21(25)27-13-28-22(18)31/h3-5,7-8,13-15,17,19-20,23,32-33H,6,9-12H2,1-2H3,(H,26,34)(H2,25,27,28)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50396981

(CHEMBL2171174)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H22N6O5S/c1-17-12-9-13(19-5-18-12)21(6-20-9)14-11(23)10(22)8(26-14)4-27-3-2-7(16)15(24)25/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H,24,25)(H,17,18,19)/t7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PRMT1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50294573

((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-((2-amin...)Show SMILES NCCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3S/c13-1-2-22-3-6-8(19)9(20)12(21-6)18-5-17-7-10(14)15-4-16-11(7)18/h4-6,8-9,12,19-20H,1-3,13H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397009
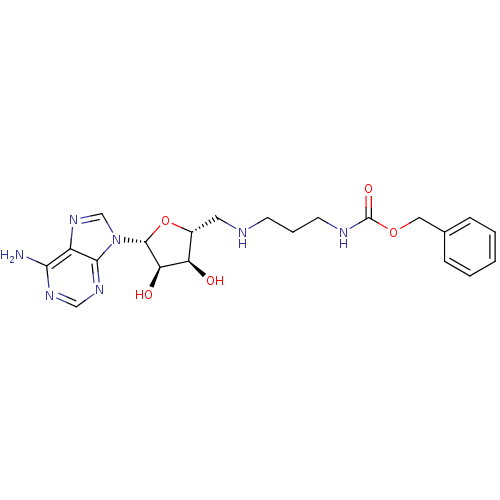
(CHEMBL2170976)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CNCCCNC(=O)OCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H27N7O5/c22-18-15-19(26-11-25-18)28(12-27-15)20-17(30)16(29)14(33-20)9-23-7-4-8-24-21(31)32-10-13-5-2-1-3-6-13/h1-3,5-6,11-12,14,16-17,20,23,29-30H,4,7-10H2,(H,24,31)(H2,22,25,26)/t14-,16-,17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397008
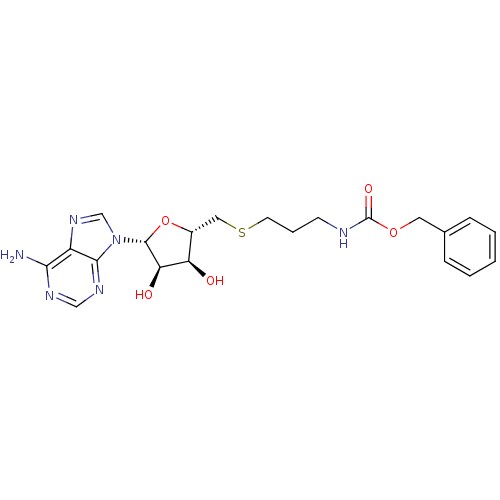
(CHEMBL2170977)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)OCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H26N6O5S/c22-18-15-19(25-11-24-18)27(12-26-15)20-17(29)16(28)14(32-20)10-33-8-4-7-23-21(30)31-9-13-5-2-1-3-6-13/h1-3,5-6,11-12,14,16-17,20,28-29H,4,7-10H2,(H,23,30)(H2,22,24,25)/t14-,16-,17-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397010

(CHEMBL2170975)Show SMILES CC(C)(C)OC(=O)NCCCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C18H28N6O5S/c1-18(2,3)29-17(27)20-5-4-6-30-7-10-12(25)13(26)16(28-10)24-9-23-11-14(19)21-8-22-15(11)24/h8-10,12-13,16,25-26H,4-7H2,1-3H3,(H,20,27)(H2,19,21,22)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50396977

(CHEMBL2171172)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C34H46N8O4/c1-22(2)41(17-9-16-35-33(45)40-25-14-12-24(13-15-25)34(3,4)5)19-26-28(43)29(44)32(46-26)42-21-39-27-30(37-20-38-31(27)42)36-18-23-10-7-6-8-11-23/h6-8,10-15,20-22,26,28-29,32,43-44H,9,16-19H2,1-5H3,(H2,35,40,45)(H,36,37,38)/t26-,28-,29-,32-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PRMT1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50396978

(CHEMBL2171171)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC=C)ncnc12 |r| Show InChI InChI=1S/C30H44N8O4/c1-7-13-31-26-23-27(34-17-33-26)38(18-35-23)28-25(40)24(39)22(42-28)16-37(19(2)3)15-8-14-32-29(41)36-21-11-9-20(10-12-21)30(4,5)6/h7,9-12,17-19,22,24-25,28,39-40H,1,8,13-16H2,2-6H3,(H,31,33,34)(H2,32,36,41)/t22-,24-,25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PRMT1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50396979

(CHEMBL2171170)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCCNC(=O)Nc2ccc(cc2)C(C)(C)C)C(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H42N8O4/c1-17(2)35(13-7-12-30-27(39)34-19-10-8-18(9-11-19)28(3,4)5)14-20-22(37)23(38)26(40-20)36-16-33-21-24(29-6)31-15-32-25(21)36/h8-11,15-17,20,22-23,26,37-38H,7,12-14H2,1-6H3,(H,29,31,32)(H2,30,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PRMT1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50396980

(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PRMT1 |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397003
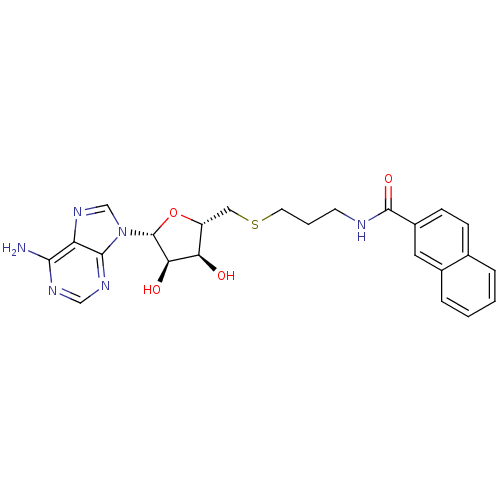
(CHEMBL2170982)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)c2ccc3ccccc3c2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H26N6O4S/c25-21-18-22(28-12-27-21)30(13-29-18)24-20(32)19(31)17(34-24)11-35-9-3-8-26-23(33)16-7-6-14-4-1-2-5-15(14)10-16/h1-2,4-7,10,12-13,17,19-20,24,31-32H,3,8-9,11H2,(H,26,33)(H2,25,27,28)/t17-,19-,20-,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50397004
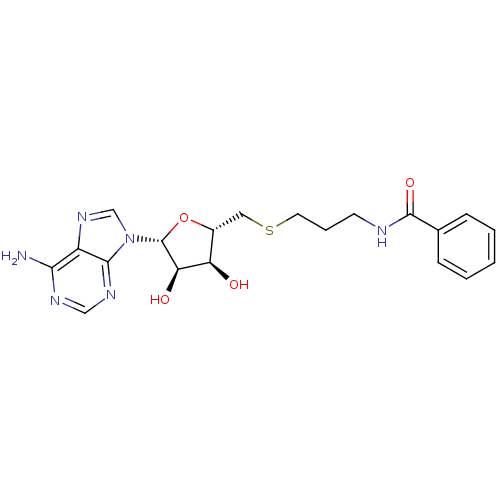
(CHEMBL2170981)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)c2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24N6O4S/c21-17-14-18(24-10-23-17)26(11-25-14)20-16(28)15(27)13(30-20)9-31-8-4-7-22-19(29)12-5-2-1-3-6-12/h1-3,5-6,10-11,13,15-16,20,27-28H,4,7-9H2,(H,22,29)(H2,21,23,24)/t13-,15-,16-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data