 Found 69 hits Enz. Inhib. hit(s) with all data for entry = 50040685
Found 69 hits Enz. Inhib. hit(s) with all data for entry = 50040685 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358609
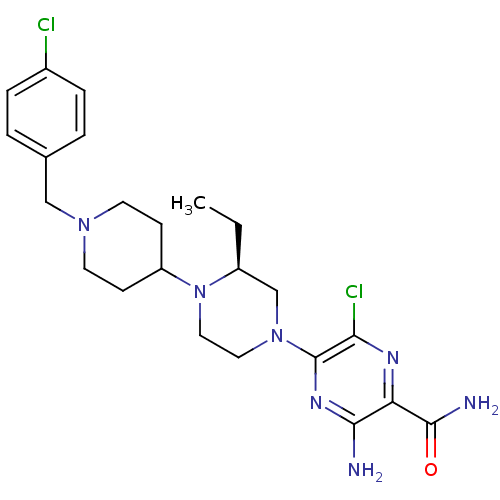
(CHEMBL1921858)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(N)=O |r| Show InChI InChI=1S/C23H31Cl2N7O/c1-2-17-14-31(23-20(25)28-19(22(27)33)21(26)29-23)11-12-32(17)18-7-9-30(10-8-18)13-15-3-5-16(24)6-4-15/h3-6,17-18H,2,7-14H2,1H3,(H2,26,29)(H2,27,33)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175523
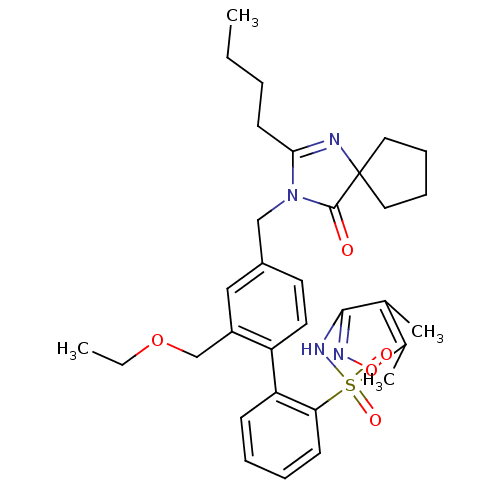
(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(COCC)c1)-c1ccccc1S(=O)(=O)Nc1noc(C)c1C |t:4| Show InChI InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50398339
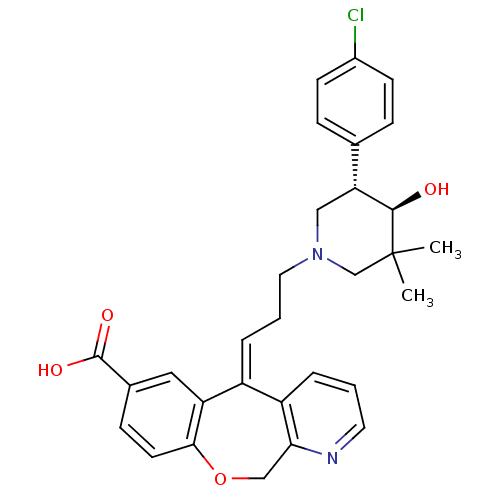
(CHEMBL2178569)Show SMILES CC1(C)CN(CC\C=C2/c3cccnc3COc3ccc(cc23)C(O)=O)C[C@@H]([C@H]1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H31ClN2O4/c1-30(2)18-33(16-25(28(30)34)19-7-10-21(31)11-8-19)14-4-6-22-23-5-3-13-32-26(23)17-37-27-12-9-20(29(35)36)15-24(22)27/h3,5-13,15,25,28,34H,4,14,16-18H2,1-2H3,(H,35,36)/b22-6+/t25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208999
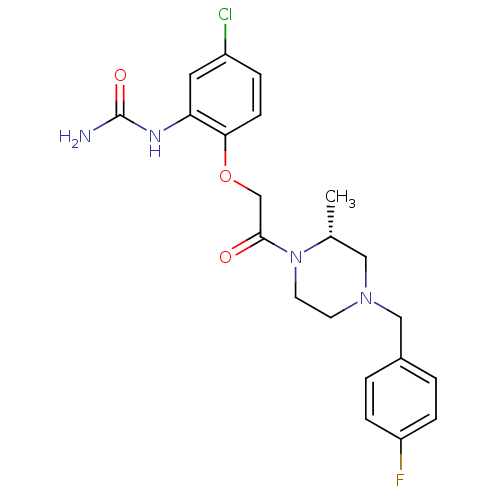
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O |r| Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50398339

(CHEMBL2178569)Show SMILES CC1(C)CN(CC\C=C2/c3cccnc3COc3ccc(cc23)C(O)=O)C[C@@H]([C@H]1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H31ClN2O4/c1-30(2)18-33(16-25(28(30)34)19-7-10-21(31)11-8-19)14-4-6-22-23-5-3-13-32-26(23)17-37-27-12-9-20(29(35)36)15-24(22)27/h3,5-13,15,25,28,34H,4,14,16-18H2,1-2H3,(H,35,36)/b22-6+/t25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to ETA receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398345
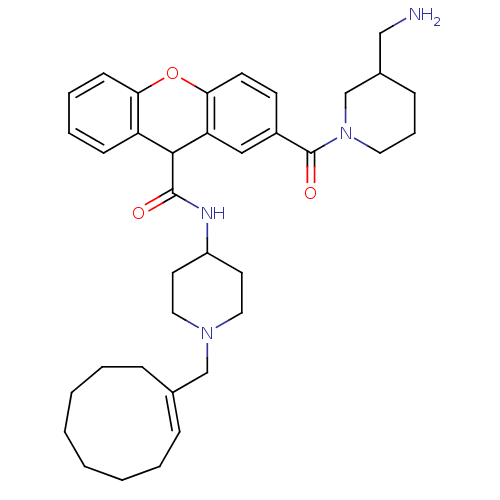
(CHEMBL2178571)Show SMILES NCC1CCCN(C1)C(=O)c1ccc2Oc3ccccc3C(C(=O)NC3CCN(C\C4=C\CCCCCCC4)CC3)c2c1 |t:32| Show InChI InChI=1S/C36H48N4O3/c37-23-27-12-9-19-40(25-27)36(42)28-15-16-33-31(22-28)34(30-13-7-8-14-32(30)43-33)35(41)38-29-17-20-39(21-18-29)24-26-10-5-3-1-2-4-6-11-26/h7-8,10,13-16,22,27,29,34H,1-6,9,11-12,17-21,23-25,37H2,(H,38,41)/b26-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50398349
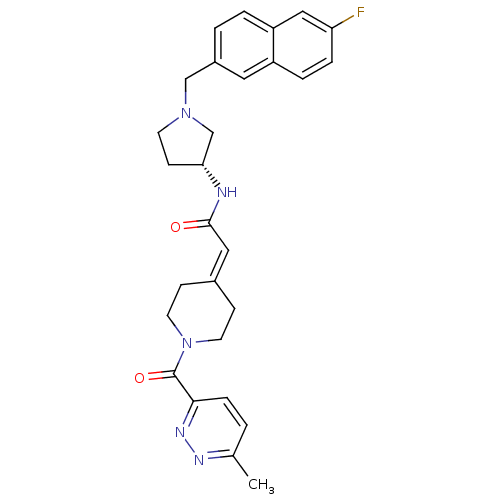
(CHEMBL2178580)Show SMILES [#6]-c1ccc(nn1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]\[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C28H30FN5O2/c1-19-2-7-26(32-31-19)28(36)34-12-8-20(9-13-34)15-27(35)30-25-10-11-33(18-25)17-21-3-4-23-16-24(29)6-5-22(23)14-21/h2-7,14-16,25H,8-13,17-18H2,1H3,(H,30,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 assessed as inhibition of CCL11-induced calcium influx by cell based assay |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50398340
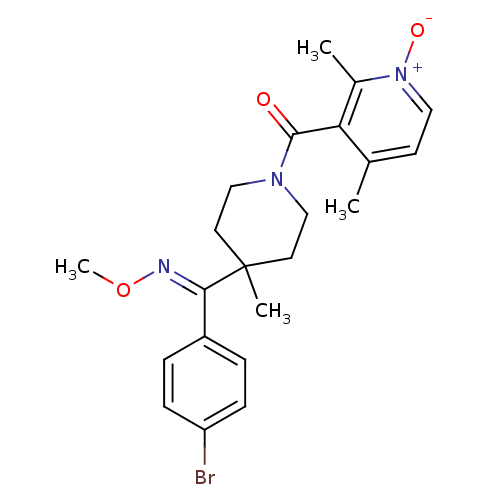
(CHEMBL2178576)Show SMILES CO\N=C(/c1ccc(Br)cc1)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C Show InChI InChI=1S/C22H26BrN3O3/c1-15-9-12-26(28)16(2)19(15)21(27)25-13-10-22(3,11-14-25)20(24-29-4)17-5-7-18(23)8-6-17/h5-9,12H,10-11,13-14H2,1-4H3/b24-20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398339

(CHEMBL2178569)Show SMILES CC1(C)CN(CC\C=C2/c3cccnc3COc3ccc(cc23)C(O)=O)C[C@@H]([C@H]1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H31ClN2O4/c1-30(2)18-33(16-25(28(30)34)19-7-10-21(31)11-8-19)14-4-6-22-23-5-3-13-32-26(23)17-37-27-12-9-20(29(35)36)15-24(22)27/h3,5-13,15,25,28,34H,4,14,16-18H2,1-2H3,(H,35,36)/b22-6+/t25-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM25351

(N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...)Show SMILES Cc1nnc(o1)C(=O)NC(C)(C)c1nc(C(=O)NCc2ccc(F)cc2)c(O)c(=O)n1C Show InChI InChI=1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398346
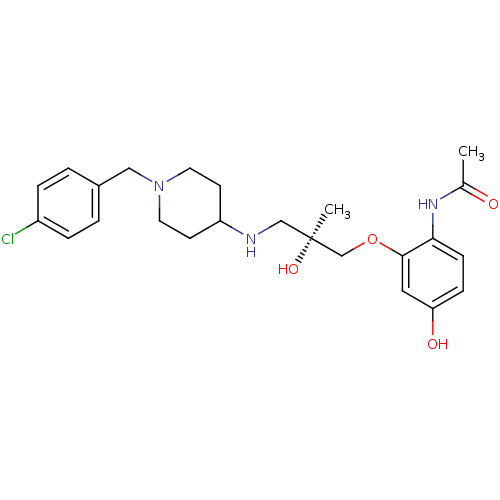
(CHEMBL2178570)Show SMILES CC(=O)Nc1ccc(O)cc1OC[C@@](C)(O)CNC1CCN(Cc2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C24H32ClN3O4/c1-17(29)27-22-8-7-21(30)13-23(22)32-16-24(2,31)15-26-20-9-11-28(12-10-20)14-18-3-5-19(25)6-4-18/h3-8,13,20,26,30-31H,9-12,14-16H2,1-2H3,(H,27,29)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50175523

(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(COCC)c1)-c1ccccc1S(=O)(=O)Nc1noc(C)c1C |t:4| Show InChI InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to ETA receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50398341
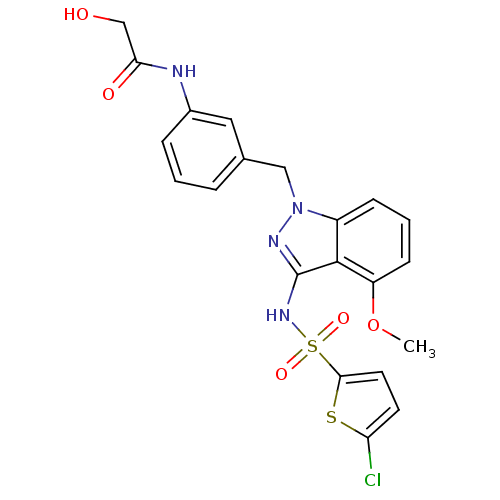
(CHEMBL2178575)Show SMILES COc1cccc2n(Cc3cccc(NC(=O)CO)c3)nc(NS(=O)(=O)c3ccc(Cl)s3)c12 Show InChI InChI=1S/C21H19ClN4O5S2/c1-31-16-7-3-6-15-20(16)21(25-33(29,30)19-9-8-17(22)32-19)24-26(15)11-13-4-2-5-14(10-13)23-18(28)12-27/h2-10,27H,11-12H2,1H3,(H,23,28)(H,24,25) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR4 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50002088
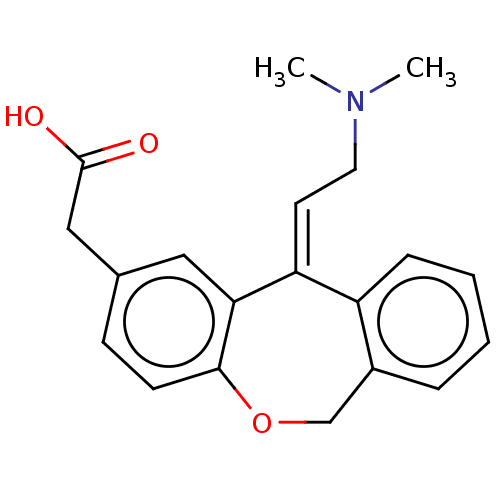
(CHEMBL302005 | [11-(2-Dimethylamino-ethylidene)-6,...)Show InChI InChI=1S/C20H21NO3/c1-21(2)10-9-17-16-6-4-3-5-15(16)13-24-19-8-7-14(11-18(17)19)12-20(22)23/h3-9,11H,10,12-13H2,1-2H3,(H,22,23)/b17-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50398348

(CHEMBL2178581)Show SMILES OC(=O)Cc1ccc2OCc3ccccc3\C(=C/Cn3cnc4ccccc34)c2c1 Show InChI InChI=1S/C25H20N2O3/c28-25(29)14-17-9-10-24-21(13-17)20(19-6-2-1-5-18(19)15-30-24)11-12-27-16-26-22-7-3-4-8-23(22)27/h1-11,13,16H,12,14-15H2,(H,28,29)/b20-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to TXA2 receptor receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50398347
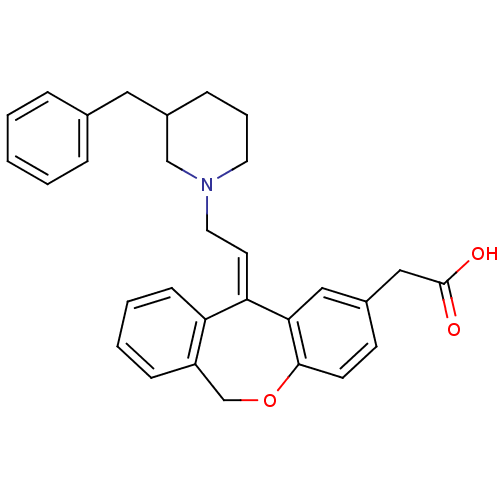
(CHEMBL2178582)Show SMILES OC(=O)Cc1ccc2OCc3ccccc3\C(=C/CN3CCCC(Cc4ccccc4)C3)c2c1 Show InChI InChI=1S/C30H31NO3/c32-30(33)19-23-12-13-29-28(18-23)27(26-11-5-4-10-25(26)21-34-29)14-16-31-15-6-9-24(20-31)17-22-7-2-1-3-8-22/h1-5,7-8,10-14,18,24H,6,9,15-17,19-21H2,(H,32,33)/b27-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50398349

(CHEMBL2178580)Show SMILES [#6]-c1ccc(nn1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]\[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C28H30FN5O2/c1-19-2-7-26(32-31-19)28(36)34-12-8-20(9-13-34)15-27(35)30-25-10-11-33(18-25)17-21-3-4-23-16-24(29)6-5-22(23)14-21/h2-7,14-16,25H,8-13,17-18H2,1H3,(H,30,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor expressed in human PC3 cells inhibition of histamine-induced calcium influx by cell based assay |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50398338
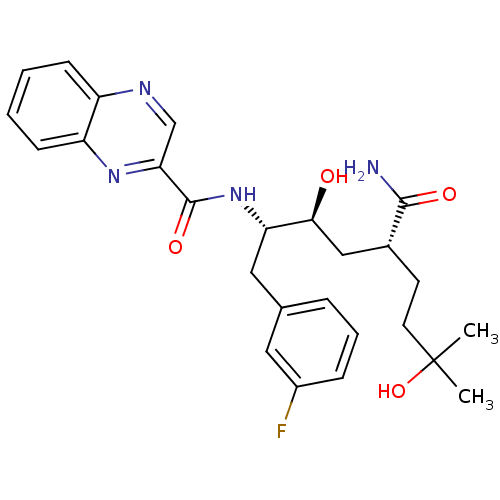
(CHEMBL1628706)Show SMILES CC(C)(O)CC[C@H](C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O |r| Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17-,21+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398338

(CHEMBL1628706)Show SMILES CC(C)(O)CC[C@H](C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O |r| Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50398347

(CHEMBL2178582)Show SMILES OC(=O)Cc1ccc2OCc3ccccc3\C(=C/CN3CCCC(Cc4ccccc4)C3)c2c1 Show InChI InChI=1S/C30H31NO3/c32-30(33)19-23-12-13-29-28(18-23)27(26-11-5-4-10-25(26)21-34-29)14-16-31-15-6-9-24(20-31)17-22-7-2-1-3-8-22/h1-5,7-8,10-14,18,24H,6,9,15-17,19-21H2,(H,32,33)/b27-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to TXA2 receptor receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50002088

(CHEMBL302005 | [11-(2-Dimethylamino-ethylidene)-6,...)Show InChI InChI=1S/C20H21NO3/c1-21(2)10-9-17-16-6-4-3-5-15(16)13-24-19-8-7-14(11-18(17)19)12-20(22)23/h3-9,11H,10,12-13H2,1-2H3,(H,22,23)/b17-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to TXA2 receptor receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50398338

(CHEMBL1628706)Show SMILES CC(C)(O)CC[C@H](C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O |r| Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17-,21+,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50091105

(4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2)-c2ncco2)c1C Show InChI InChI=1S/C20H17N3O4S/c1-13-14(2)22-27-19(13)23-28(24,25)18-6-4-3-5-17(18)15-7-9-16(10-8-15)20-21-11-12-26-20/h3-12,23H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to ETA receptor |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 assessed as inhibition of chemotaxis by cell based assay |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200880
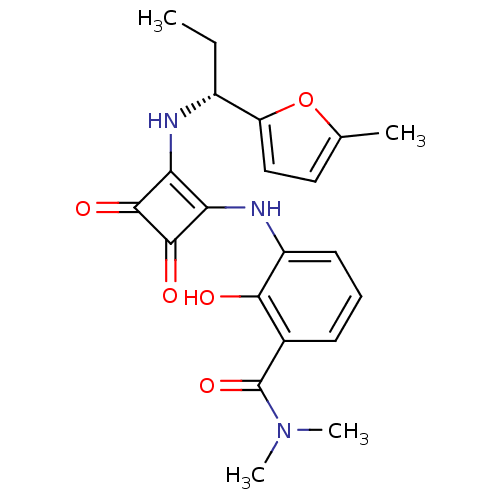
((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)o1 |r| Show InChI InChI=1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR2 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50398336
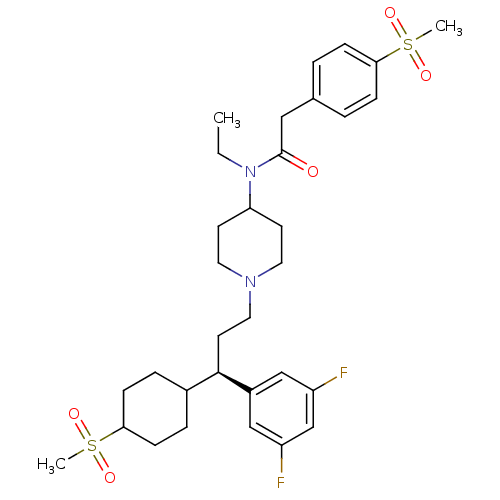
(CHEMBL2178577)Show SMILES CCN(C1CCN(CC[C@H](C2CCC(CC2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r,wU:9.8,(42.25,-25.89,;43.58,-26.67,;43.58,-28.21,;42.24,-28.98,;42.23,-30.52,;40.9,-31.28,;39.57,-30.51,;38.24,-31.28,;36.9,-30.51,;35.57,-31.28,;34.24,-30.51,;34.25,-28.97,;32.91,-28.19,;31.58,-28.97,;31.58,-30.51,;32.92,-31.27,;30.24,-28.19,;28.9,-28.97,;29.46,-26.85,;31,-26.85,;35.57,-32.82,;36.9,-33.58,;36.9,-35.12,;38.24,-35.88,;35.57,-35.89,;34.23,-35.13,;32.9,-35.89,;34.23,-33.59,;39.56,-28.97,;40.9,-28.2,;44.91,-28.99,;44.9,-30.53,;46.24,-28.22,;47.57,-29,;47.56,-30.53,;48.89,-31.31,;50.23,-30.54,;50.23,-28.99,;48.9,-28.22,;51.56,-31.31,;52.9,-30.55,;50.77,-32.64,;52.31,-32.64,)| Show InChI InChI=1S/C32H44F2N2O5S2/c1-4-36(32(37)19-23-5-9-29(10-6-23)42(2,38)39)28-13-16-35(17-14-28)18-15-31(25-20-26(33)22-27(34)21-25)24-7-11-30(12-8-24)43(3,40)41/h5-6,9-10,20-22,24,28,30-31H,4,7-8,11-19H2,1-3H3/t24?,30?,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50185666
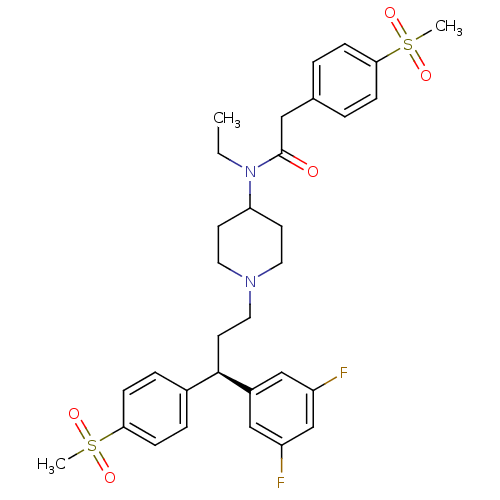
((R)-N-(1-(3-(3,5-difluorophenyl)-3-(4-(methylsulfo...)Show SMILES CCN(C1CCN(CC[C@H](c2ccc(cc2)S(C)(=O)=O)c2cc(F)cc(F)c2)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H38F2N2O5S2/c1-4-36(32(37)19-23-5-9-29(10-6-23)42(2,38)39)28-13-16-35(17-14-28)18-15-31(25-20-26(33)22-27(34)21-25)24-7-11-30(12-8-24)43(3,40)41/h5-12,20-22,28,31H,4,13-19H2,1-3H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50145685
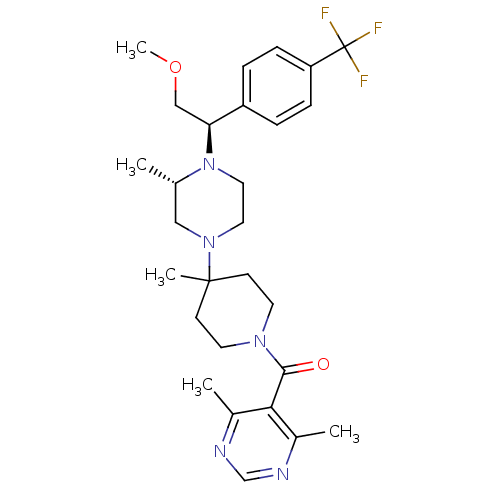
((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...)Show SMILES COC[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H38F3N5O2/c1-19-16-35(14-15-36(19)24(17-38-5)22-6-8-23(9-7-22)28(29,30)31)27(4)10-12-34(13-11-27)26(37)25-20(2)32-18-33-21(25)3/h6-9,18-19,24H,10-17H2,1-5H3/t19-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50169045
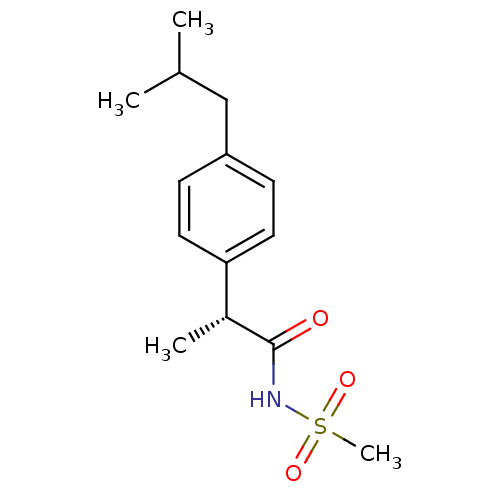
((R)-2-(4-isobutylphenyl)-N-(methylsulfonyl)propana...)Show InChI InChI=1S/C14H21NO3S/c1-10(2)9-12-5-7-13(8-6-12)11(3)14(16)15-19(4,17)18/h5-8,10-11H,9H2,1-4H3,(H,15,16)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CXCR2 assessed as inhibition of CXCL8-induced neutrophil chemotaxis |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50169045

((R)-2-(4-isobutylphenyl)-N-(methylsulfonyl)propana...)Show InChI InChI=1S/C14H21NO3S/c1-10(2)9-12-5-7-13(8-6-12)11(3)14(16)15-19(4,17)18/h5-8,10-11H,9H2,1-4H3,(H,15,16)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR3 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50398334
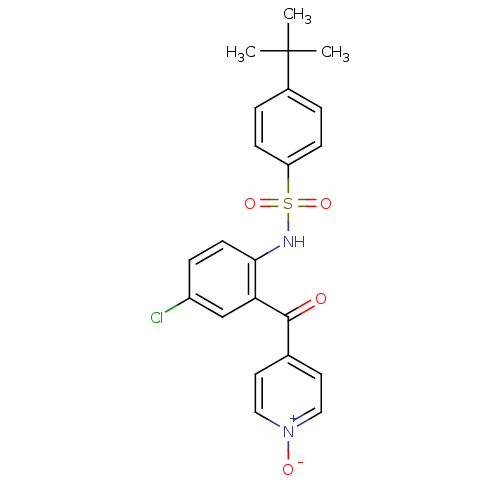
(CHEMBL2178578)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1C(=O)c1cc[n+]([O-])cc1 Show InChI InChI=1S/C22H21ClN2O4S/c1-22(2,3)16-4-7-18(8-5-16)30(28,29)24-20-9-6-17(23)14-19(20)21(26)15-10-12-25(27)13-11-15/h4-14,24H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR9 assessed as inhibition of CCL25-induced chemotaxis by cell based assay |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50422828
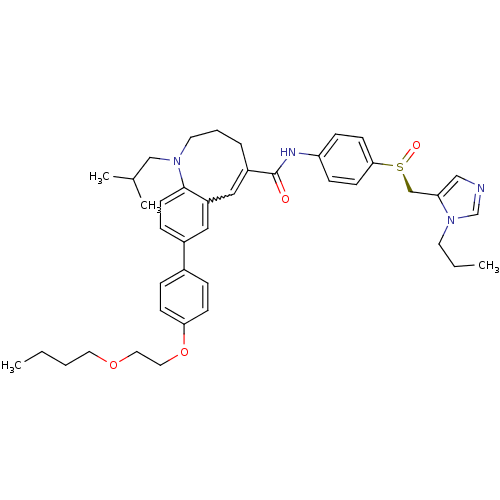
(CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...)Show SMILES CCCCOCCOc1ccc(cc1)-c1ccc2N(CC(C)C)CCCC(=Cc2c1)C(=O)Nc1ccc(cc1)[S@@](=O)Cc1cncn1CCC |r,w:27.28| Show InChI InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/t50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 assessed as inhibition of CCL3 binding |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50334986

(4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...)Show SMILES CC(C)c1nnc(C)n1[C@H]1C[C@@H]2CC[C@H](C1)N2CC[C@H](NC(=O)C1CCC(F)(F)CC1)c1ccccc1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50339033
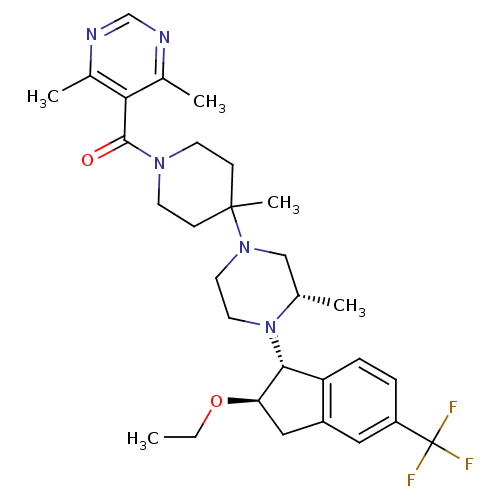
(5-[(4-(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)...)Show SMILES CCO[C@@H]1Cc2cc(ccc2[C@H]1N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)C(F)(F)F |r| Show InChI InChI=1S/C30H40F3N5O2/c1-6-40-25-16-22-15-23(30(31,32)33)7-8-24(22)27(25)38-14-13-37(17-19(38)2)29(5)9-11-36(12-10-29)28(39)26-20(3)34-18-35-21(26)4/h7-8,15,18-19,25,27H,6,9-14,16-17H2,1-5H3/t19-,25+,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR5 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398339

(CHEMBL2178569)Show SMILES CC1(C)CN(CC\C=C2/c3cccnc3COc3ccc(cc23)C(O)=O)C[C@@H]([C@H]1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H31ClN2O4/c1-30(2)18-33(16-25(28(30)34)19-7-10-21(31)11-8-19)14-4-6-22-23-5-3-13-32-26(23)17-37-27-12-9-20(29(35)36)15-24(22)27/h3,5-13,15,25,28,34H,4,14,16-18H2,1-2H3,(H,35,36)/b22-6+/t25-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 assessed as inhibition of CCL3-induced chemotaxis by cell based assay |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50381177
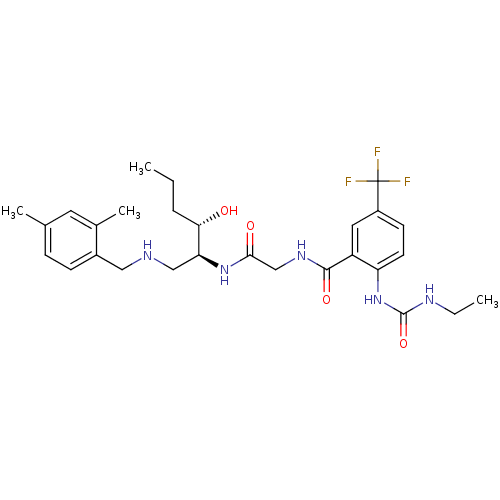
(CHEMBL2018386)Show SMILES CCC[C@H](O)[C@H](CNCc1ccc(C)cc1C)NC(=O)CNC(=O)c1cc(ccc1NC(=O)NCC)C(F)(F)F |r| Show InChI InChI=1S/C28H38F3N5O4/c1-5-7-24(37)23(15-32-14-19-9-8-17(3)12-18(19)4)35-25(38)16-34-26(39)21-13-20(28(29,30)31)10-11-22(21)36-27(40)33-6-2/h8-13,23-24,32,37H,5-7,14-16H2,1-4H3,(H,34,39)(H,35,38)(H2,33,36,40)/t23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR2 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50200880

((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccc(C)o1 |r| Show InChI InChI=1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50398343
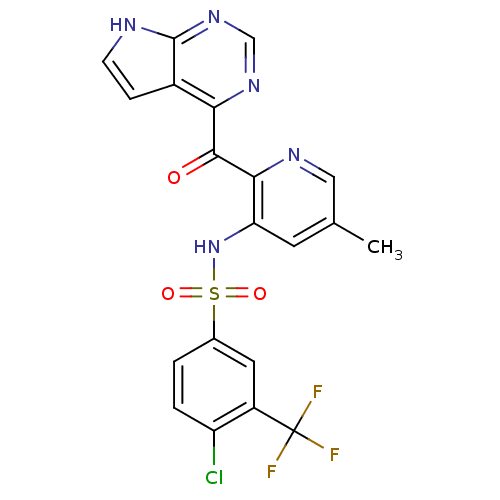
(CHEMBL2178573 | US9394307, 4-chloro-N-(5-methyl-2-...)Show SMILES Cc1cnc(C(=O)c2ncnc3[nH]ccc23)c(NS(=O)(=O)c2ccc(Cl)c(c2)C(F)(F)F)c1 Show InChI InChI=1S/C20H13ClF3N5O3S/c1-10-6-15(29-33(31,32)11-2-3-14(21)13(7-11)20(22,23)24)17(26-8-10)18(30)16-12-4-5-25-19(12)28-9-27-16/h2-9,29H,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR2 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398344
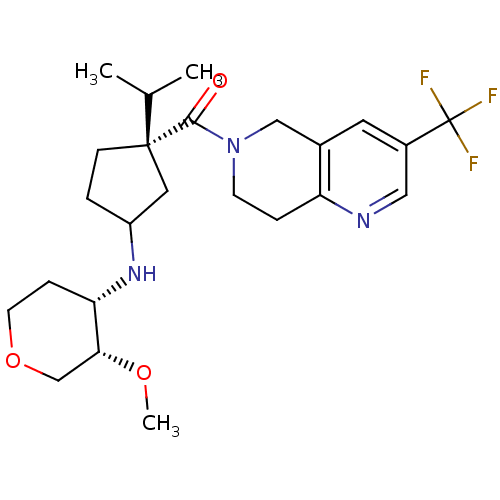
(CHEMBL2178572)Show SMILES CO[C@@H]1COCC[C@@H]1NC1CC[C@](C1)(C(C)C)C(=O)N1CCc2ncc(cc2C1)C(F)(F)F |r| Show InChI InChI=1S/C24H34F3N3O3/c1-15(2)23(7-4-18(11-23)29-20-6-9-33-14-21(20)32-3)22(31)30-8-5-19-16(13-30)10-17(12-28-19)24(25,26)27/h10,12,15,18,20-21,29H,4-9,11,13-14H2,1-3H3/t18?,20-,21+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50398333

(CHEMBL2178579)Show SMILES Oc1c(NC(=O)Nc2cccc(F)c2Cl)ccc(Cl)c1S(=O)(=O)N1CCNCC1 Show InChI InChI=1S/C17H17Cl2FN4O4S/c18-10-4-5-13(23-17(26)22-12-3-1-2-11(20)14(12)19)15(25)16(10)29(27,28)24-8-6-21-7-9-24/h1-5,21,25H,6-9H2,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR2 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50422828

(CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...)Show SMILES CCCCOCCOc1ccc(cc1)-c1ccc2N(CC(C)C)CCCC(=Cc2c1)C(=O)Nc1ccc(cc1)[S@@](=O)Cc1cncn1CCC |r,w:27.28| Show InChI InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/t50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 assessed as inhibition of CCL2 binding |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 9
(Homo sapiens (Human)) | BDBM50398334

(CHEMBL2178578)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(Cl)cc1C(=O)c1cc[n+]([O-])cc1 Show InChI InChI=1S/C22H21ClN2O4S/c1-22(2,3)16-4-7-18(8-5-16)30(28,29)24-20-9-6-17(23)14-19(20)21(26)15-10-12-25(27)13-11-15/h4-14,24H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CCR9 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394155
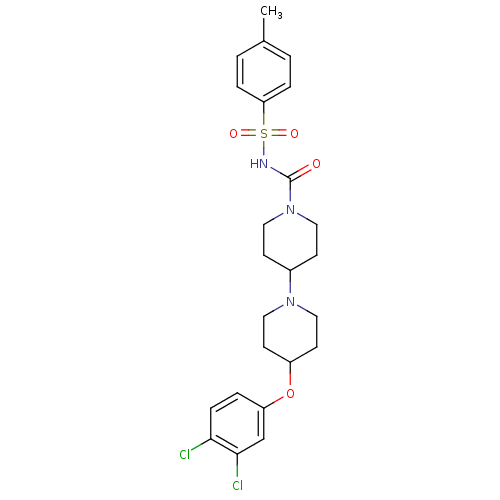
(CHEMBL2158814)Show SMILES Cc1ccc(cc1)S(=O)(=O)NC(=O)N1CCC(CC1)N1CCC(CC1)Oc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C24H29Cl2N3O4S/c1-17-2-5-21(6-3-17)34(31,32)27-24(30)29-12-8-18(9-13-29)28-14-10-19(11-15-28)33-20-4-7-22(25)23(26)16-20/h2-7,16,18-19H,8-15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 assessed as inhibition of eotaxin binding |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50398350
(CHEMBL486030 | UCB-35625)Show SMILES CC[N+]1(CC2CCCCCC2)CCC(CC1)NC(=O)C1c2cc(Cl)ccc2Oc2ccc(Cl)cc12 |(6.24,.37,;4.7,.37,;3.93,-.96,;3.15,.37,;1.61,.36,;.95,-1.01,;-.55,-1.37,;-1.76,-.42,;-1.77,1.11,;-.58,2.08,;.93,1.75,;5.26,-1.74,;5.27,-3.27,;3.93,-4.04,;2.6,-3.28,;2.6,-1.74,;3.94,-5.58,;2.61,-6.36,;1.27,-5.59,;2.62,-7.9,;3.95,-8.66,;5.28,-7.88,;6.62,-8.64,;7.94,-7.85,;6.63,-10.19,;5.3,-10.97,;3.96,-10.2,;2.63,-10.98,;1.29,-10.21,;-.04,-10.99,;-1.37,-10.22,;-1.37,-8.68,;-2.7,-7.91,;-.04,-7.91,;1.29,-8.67,)| Show InChI InChI=1S/C29H36Cl2N2O2/c1-2-33(19-20-7-5-3-4-6-8-20)15-13-23(14-16-33)32-29(34)28-24-17-21(30)9-11-26(24)35-27-12-10-22(31)18-25(27)28/h9-12,17-18,20,23,28H,2-8,13-16,19H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 assessed as inhibition of CCL3-induced chemotaxis by cell based assay |
J Med Chem 55: 9363-92 (2012)
Article DOI: 10.1021/jm300682j
BindingDB Entry DOI: 10.7270/Q2862HKR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data