 Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50040832
Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50040832 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50400807
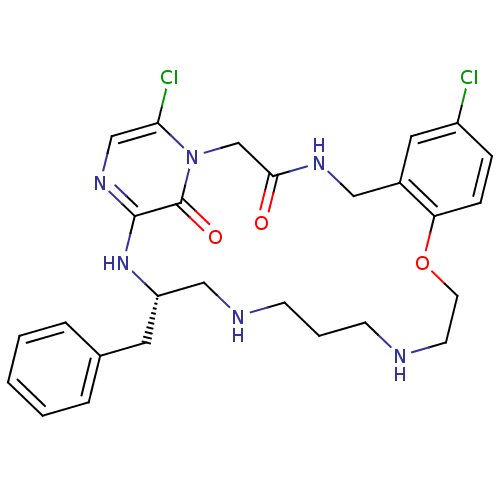
(CHEMBL2204324)Show SMILES Clc1ccc2OCCNCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |r| Show InChI InChI=1S/C27H32Cl2N6O3/c28-21-7-8-23-20(14-21)15-32-25(36)18-35-24(29)17-33-26(27(35)37)34-22(13-19-5-2-1-3-6-19)16-31-10-4-9-30-11-12-38-23/h1-3,5-8,14,17,22,30-31H,4,9-13,15-16,18H2,(H,32,36)(H,33,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50066331
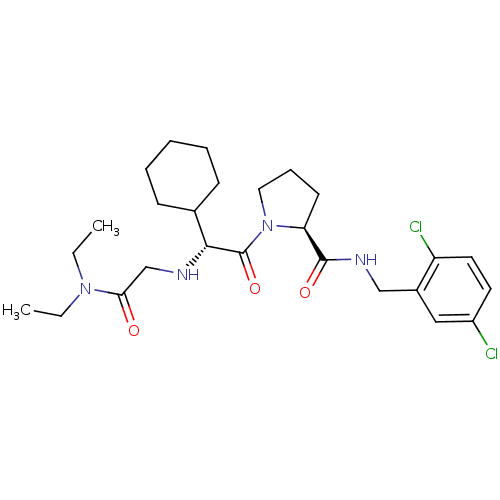
((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...)Show SMILES CCN(CC)C(=O)CN[C@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1Cl Show InChI InChI=1S/C26H38Cl2N4O3/c1-3-31(4-2)23(33)17-29-24(18-9-6-5-7-10-18)26(35)32-14-8-11-22(32)25(34)30-16-19-15-20(27)12-13-21(19)28/h12-13,15,18,22,24,29H,3-11,14,16-17H2,1-2H3,(H,30,34)/t22-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50331669
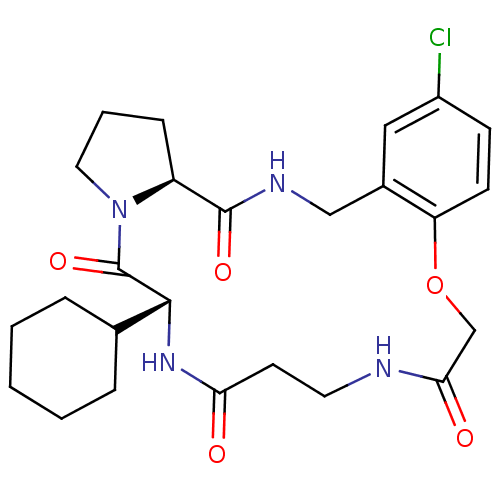
((6R,21AS)-17-CHLORO-6-CYCLOHEXYL-2,3,6,7,10,11,19,...)Show SMILES Clc1ccc2OCC(=O)NCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 |r| Show InChI InChI=1S/C25H33ClN4O5/c26-18-8-9-20-17(13-18)14-28-24(33)19-7-4-12-30(19)25(34)23(16-5-2-1-3-6-16)29-21(31)10-11-27-22(32)15-35-20/h8-9,13,16,19,23H,1-7,10-12,14-15H2,(H,27,32)(H,28,33)(H,29,31)/t19-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50400806
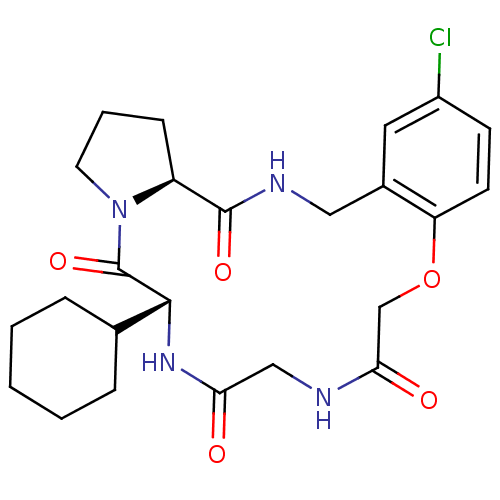
(CHEMBL2204325)Show SMILES Clc1ccc2OCC(=O)NCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 |r| Show InChI InChI=1S/C24H31ClN4O5/c25-17-8-9-19-16(11-17)12-27-23(32)18-7-4-10-29(18)24(33)22(15-5-2-1-3-6-15)28-20(30)13-26-21(31)14-34-19/h8-9,11,15,18,22H,1-7,10,12-14H2,(H,26,31)(H,27,32)(H,28,30)/t18-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131468

((S)-24-Chloro-11-(R)-cyclohexyl-20-oxa-3,9,12,17-t...)Show SMILES Clc1ccc2OCC(=O)NCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C26H35ClN4O5/c27-19-10-11-21-18(14-19)15-29-25(34)20-8-5-13-31(20)26(35)24(17-6-2-1-3-7-17)30-22(32)9-4-12-28-23(33)16-36-21/h10-11,14,17,20,24H,1-9,12-13,15-16H2,(H,28,33)(H,29,34)(H,30,32)/t20-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131463
((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...)Show SMILES Clc1ccc2OCC(=O)NCCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1 Show InChI InChI=1S/C27H37ClN4O5/c28-20-11-12-22-19(15-20)16-30-26(35)21-9-6-14-32(21)27(36)25(18-7-2-1-3-8-18)31-23(33)10-4-5-13-29-24(34)17-37-22/h11-12,15,18,21,25H,1-10,13-14,16-17H2,(H,29,34)(H,30,35)(H,31,33)/t21-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50359261
(CHEMBL1923617)Show SMILES CC[C@H](C)[C@@H]1NCCOc2ccccc2CCCNC(=O)[C@@H](Cc2ccc(Cl)cc2)NC(=O)[C@@H](C)N(C)C1=O |r| Show InChI InChI=1S/C30H41ClN4O4/c1-5-20(2)27-30(38)35(4)21(3)28(36)34-25(19-22-12-14-24(31)15-13-22)29(37)33-16-8-10-23-9-6-7-11-26(23)39-18-17-32-27/h6-7,9,11-15,20-21,25,27,32H,5,8,10,16-19H2,1-4H3,(H,33,37)(H,34,36)/t20-,21+,25+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Agonist activity at ghrelin receptor by cell based assay |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50359282
(CHEMBL1923609)Show SMILES CC[C@H](C)[C@@H]1NCCOc2ccccc2CCCNC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)N(C)C1=O |r| Show InChI InChI=1S/C30H42N4O4/c1-5-21(2)27-30(37)34(4)22(3)28(35)33-25(20-23-12-7-6-8-13-23)29(36)32-17-11-15-24-14-9-10-16-26(24)38-19-18-31-27/h6-10,12-14,16,21-22,25,27,31H,5,11,15,17-20H2,1-4H3,(H,32,36)(H,33,35)/t21-,22+,25+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Agonist activity at ghrelin receptor by cell based assay |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50400799

(CHEMBL2203623)Show SMILES CC[C@H](C)[C@@H]1NCCOc2ccccc2CCCNC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CO)N(C)C1=O |r| Show InChI InChI=1S/C30H42N4O5/c1-4-21(2)27-30(38)34(3)25(20-35)29(37)33-24(19-22-11-6-5-7-12-22)28(36)32-16-10-14-23-13-8-9-15-26(23)39-18-17-31-27/h5-9,11-13,15,21,24-25,27,31,35H,4,10,14,16-20H2,1-3H3,(H,32,36)(H,33,37)/t21-,24+,25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Agonist activity at ghrelin receptor |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118723
(CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-1=O)-[#7]-[#6]=O |r,t:24| Show InChI InChI=1S/C36H45N9O8/c37-36(38)39-16-4-8-26-31(49)34(52)44-27(19-22-6-2-1-3-7-22)32(50)42-24(18-23-10-13-25(47)14-11-23)12-15-30(48)40-20-28(41-21-46)35(53)45-17-5-9-29(45)33(51)43-26/h1-3,6-7,10-15,21,24,26-29,47H,4-5,8-9,16-20H2,(H,40,48)(H,41,46)(H,42,50)(H,43,51)(H,44,52)(H4,37,38,39)/b15-12+/t24-,26+,27-,28+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50400811
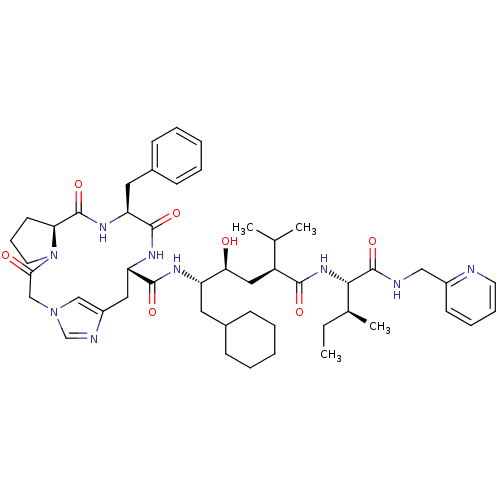
(CHEMBL2204329)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H]1Cc2cn(CC(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(=O)N1)cn2)C(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C49H69N9O7/c1-5-32(4)44(49(65)51-27-35-19-12-13-21-50-35)56-45(61)37(31(2)3)26-42(59)38(23-33-15-8-6-9-16-33)53-47(63)40-25-36-28-57(30-52-36)29-43(60)58-22-14-20-41(58)48(64)55-39(46(62)54-40)24-34-17-10-7-11-18-34/h7,10-13,17-19,21,28,30-33,37-42,44,59H,5-6,8-9,14-16,20,22-27,29H2,1-4H3,(H,51,65)(H,53,63)(H,54,62)(H,55,64)(H,56,61)/t32-,37-,38-,39-,40-,41-,42-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50400808
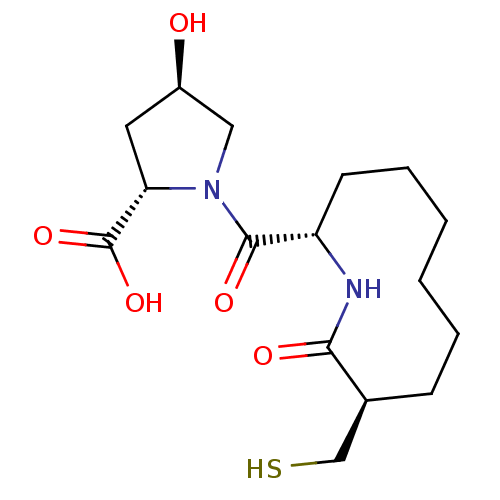
(CHEMBL2204326)Show SMILES O[C@@H]1C[C@H](N(C1)C(=O)[C@@H]1CCCCCC[C@@H](CS)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C16H26N2O5S/c19-11-7-13(16(22)23)18(8-11)15(21)12-6-4-2-1-3-5-10(9-24)14(20)17-12/h10-13,19,24H,1-9H2,(H,17,20)(H,22,23)/t10-,11+,12-,13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50400801
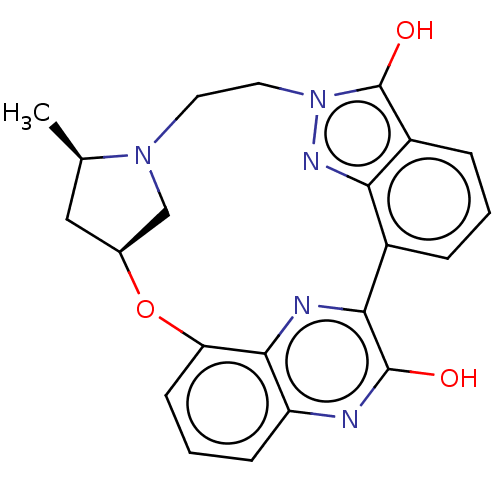
(CHEMBL215803)Show SMILES [H][C@]12C[C@@H](C)N(C1)CCn1nc3c(cccc3c1O)-c1nc3c(O2)cccc3nc1O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50400801

(CHEMBL215803)Show SMILES [H][C@]12C[C@@H](C)N(C1)CCn1nc3c(cccc3c1O)-c1nc3c(O2)cccc3nc1O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50400801

(CHEMBL215803)Show SMILES [H][C@]12C[C@@H](C)N(C1)CCn1nc3c(cccc3c1O)-c1nc3c(O2)cccc3nc1O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50400801

(CHEMBL215803)Show SMILES [H][C@]12C[C@@H](C)N(C1)CCn1nc3c(cccc3c1O)-c1nc3c(O2)cccc3nc1O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50400813
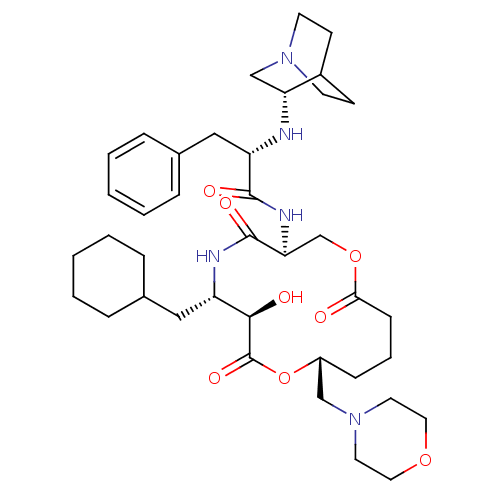
(CHEMBL2204327)Show SMILES O[C@@H]1[C@H](CC2CCCCC2)NC(=O)[C@H](COC(=O)CCC[C@H](CN2CCOCC2)OC1=O)NC(=O)[C@H](Cc1ccccc1)N[C@@H]1CN2CCC1CC2 |r,wU:13.34,wD:44.47,35.46,1.0,2.2,21.22,(24.68,-47.15,;23.35,-46.38,;22.01,-47.15,;22.01,-48.69,;23.35,-49.47,;23.34,-51,;24.67,-51.76,;26.01,-51.01,;26.01,-49.46,;24.68,-48.68,;20.68,-46.38,;19.35,-47.15,;19.35,-48.69,;18.02,-46.38,;18.02,-44.84,;16.67,-44.07,;16.67,-42.52,;15.34,-41.76,;18.02,-41.76,;19.35,-42.52,;20.68,-41.76,;22.01,-42.52,;23.35,-41.76,;23.35,-40.22,;24.68,-39.45,;24.69,-37.91,;23.36,-37.15,;22.01,-37.91,;22.01,-39.45,;22.01,-44.06,;23.35,-44.84,;24.68,-44.06,;16.67,-47.15,;15.34,-46.38,;15.34,-44.84,;14.01,-47.15,;14.01,-48.69,;15.34,-49.47,;15.33,-51.01,;16.66,-51.77,;18,-51.01,;18,-49.46,;16.66,-48.69,;12.67,-46.38,;11.34,-47.15,;11.34,-48.69,;10,-49.47,;8.67,-48.69,;8.68,-47.15,;10.01,-46.38,;9.22,-47.72,;10.7,-48.1,)| Show InChI InChI=1S/C39H59N5O8/c45-35-13-7-12-30(24-44-18-20-50-21-19-44)52-39(49)36(46)31(22-27-8-3-1-4-9-27)41-38(48)34(26-51-35)42-37(47)32(23-28-10-5-2-6-11-28)40-33-25-43-16-14-29(33)15-17-43/h2,5-6,10-11,27,29-34,36,40,46H,1,3-4,7-9,12-26H2,(H,41,48)(H,42,47)/t30-,31+,32+,33-,34+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50400805
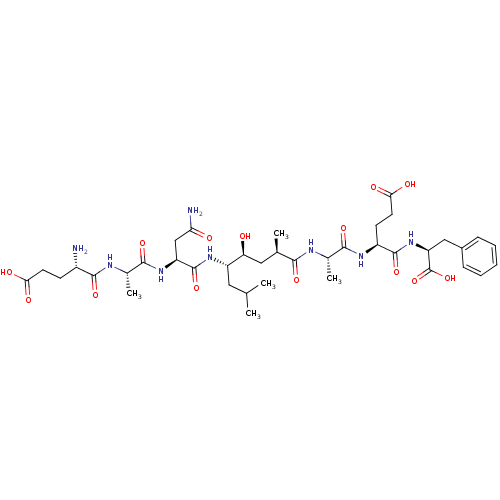
(CHEMBL2204316)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H60N8O14/c1-19(2)15-26(45-38(59)27(18-30(41)49)46-35(56)22(5)43-36(57)24(40)11-13-31(50)51)29(48)16-20(3)33(54)42-21(4)34(55)44-25(12-14-32(52)53)37(58)47-28(39(60)61)17-23-9-7-6-8-10-23/h6-10,19-22,24-29,48H,11-18,40H2,1-5H3,(H2,41,49)(H,42,54)(H,43,57)(H,44,55)(H,45,59)(H,46,56)(H,47,58)(H,50,51)(H,52,53)(H,60,61)/t20-,21+,22+,24+,25+,26+,27+,28+,29+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50056251
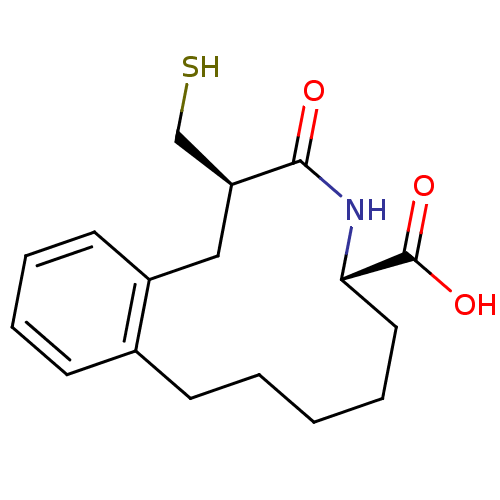
((6R,9S)-6-Mercaptomethyl-7-oxo-5,6,7,8,9,10,11,12,...)Show SMILES OC(=O)[C@@H]1CCCCCc2ccccc2C[C@@H](CS)C(=O)N1 Show InChI InChI=1S/C17H23NO3S/c19-16-14(11-22)10-13-8-5-4-7-12(13)6-2-1-3-9-15(18-16)17(20)21/h4-5,7-8,14-15,22H,1-3,6,9-11H2,(H,18,19)(H,20,21)/t14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50056258
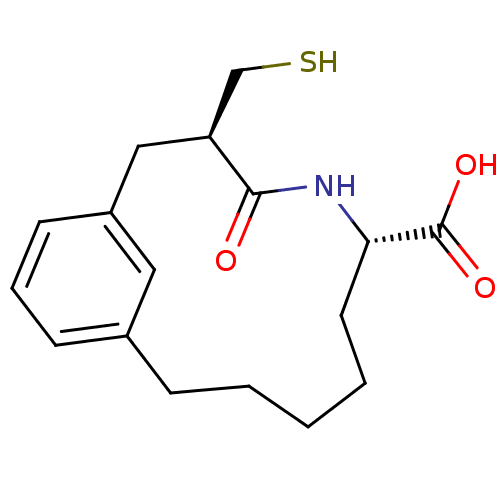
((3R,6S)-3-Mercaptomethyl-4-oxo-5-aza-bicyclo[10.3....)Show SMILES OC(=O)[C@@H]1CCCCCc2cccc(C[C@@H](CS)C(=O)N1)c2 Show InChI InChI=1S/C17H23NO3S/c19-16-14(11-22)10-13-7-4-6-12(9-13)5-2-1-3-8-15(18-16)17(20)21/h4,6-7,9,14-15,22H,1-3,5,8,10-11H2,(H,18,19)(H,20,21)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50056258

((3R,6S)-3-Mercaptomethyl-4-oxo-5-aza-bicyclo[10.3....)Show SMILES OC(=O)[C@@H]1CCCCCc2cccc(C[C@@H](CS)C(=O)N1)c2 Show InChI InChI=1S/C17H23NO3S/c19-16-14(11-22)10-13-7-4-6-12(9-13)5-2-1-3-8-15(18-16)17(20)21/h4,6-7,9,14-15,22H,1-3,5,8,10-11H2,(H,18,19)(H,20,21)/t14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50035696
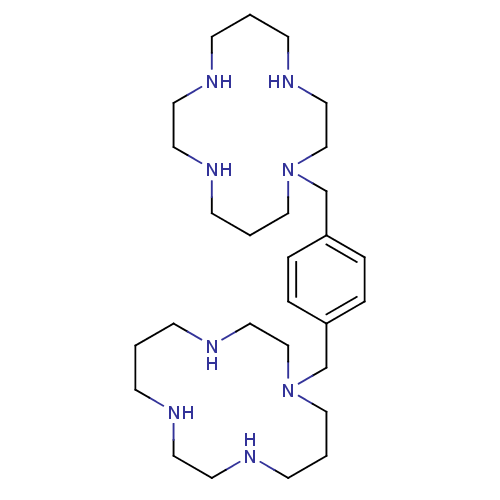
(1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...)Show SMILES C(N1CCCNCCNCCCNCC1)c1ccc(CN2CCCNCCNCCCNCC2)cc1 Show InChI InChI=1S/C28H54N8/c1-9-29-15-17-31-13-3-21-35(23-19-33-11-1)25-27-5-7-28(8-6-27)26-36-22-4-14-32-18-16-30-10-2-12-34-20-24-36/h5-8,29-34H,1-4,9-26H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Antagonist activity at CXCR4 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50270276

(CHEMBL477121 | N-[1,4,8,11-Tetraazacyclotetradecan...)Show InChI InChI=1S/C24H38N6/c1-2-13-29-24(5-1)20-28-19-22-6-8-23(9-7-22)21-30-17-4-12-26-15-14-25-10-3-11-27-16-18-30/h1-2,5-9,13,25-28H,3-4,10-12,14-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Antagonist activity at CXCR4 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50400802

(CHEMBL1235529)Show InChI InChI=1S/C13H14BrN5O2S/c14-11-8-16-13-18-9-3-1-4-10(7-9)22(20,21)17-6-2-5-15-12(11)19-13/h1,3-4,7-8,17H,2,5-6H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50212189

(2-((5R,14R)-5-amino-5-methyl-4,16-dioxo-14-phenyl-...)Show SMILES C[C@@]1(N)Cc2cccc(CC[C@@H](NC(=O)c3cc(COC1=O)cc(c3)-c1ccccc1C#N)c1ccccc1)c2 Show InChI InChI=1S/C34H31N3O3/c1-34(36)20-24-9-7-8-23(16-24)14-15-31(26-10-3-2-4-11-26)37-32(38)29-18-25(22-40-33(34)39)17-28(19-29)30-13-6-5-12-27(30)21-35/h2-13,16-19,31H,14-15,20,22,36H2,1H3,(H,37,38)/t31-,34-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50400804
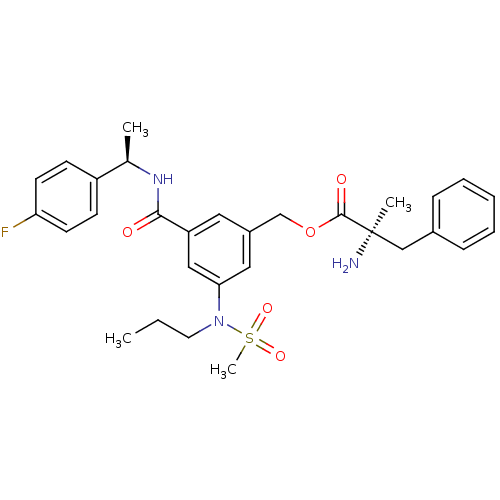
(CHEMBL2203629)Show SMILES CCCN(c1cc(COC(=O)[C@](C)(N)Cc2ccccc2)cc(c1)C(=O)N[C@H](C)c1ccc(F)cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C30H36FN3O5S/c1-5-15-34(40(4,37)38)27-17-23(20-39-29(36)30(3,32)19-22-9-7-6-8-10-22)16-25(18-27)28(35)33-21(2)24-11-13-26(31)14-12-24/h6-14,16-18,21H,5,15,19-20,32H2,1-4H3,(H,33,35)/t21-,30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50400802

(CHEMBL1235529)Show InChI InChI=1S/C13H14BrN5O2S/c14-11-8-16-13-18-9-3-1-4-10(7-9)22(20,21)17-6-2-5-15-12(11)19-13/h1,3-4,7-8,17H,2,5-6H2,(H2,15,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50400812
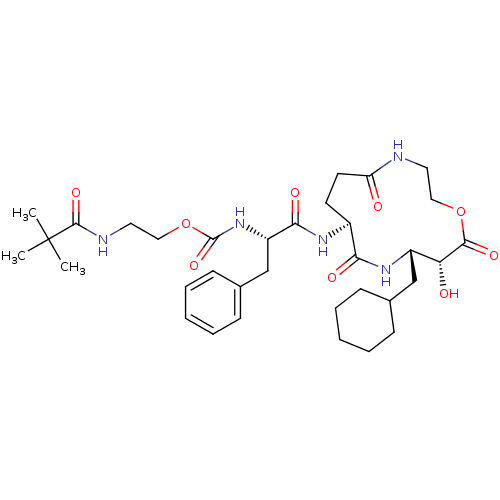
(CHEMBL2204328)Show SMILES CC(C)(C)C(=O)NCCOC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCC(=O)NCCOC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O |r| Show InChI InChI=1S/C34H51N5O9/c1-34(2,3)32(45)36-17-19-48-33(46)39-26(21-23-12-8-5-9-13-23)30(43)37-24-14-15-27(40)35-16-18-47-31(44)28(41)25(38-29(24)42)20-22-10-6-4-7-11-22/h5,8-9,12-13,22,24-26,28,41H,4,6-7,10-11,14-21H2,1-3H3,(H,35,40)(H,36,45)(H,37,43)(H,38,42)(H,39,46)/t24-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM15799

(CHEMBL81671 | Macrocyclic BACE inhibitor 1 | tert-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccncc1 |r| Show InChI InChI=1S/C37H56N6O7/c1-22(2)30(34(47)39-21-27-15-17-38-18-16-27)42-32(45)24(5)19-29(44)28(20-26-13-11-10-12-14-26)41-33(46)25(6)40-35(48)31(23(3)4)43-36(49)50-37(7,8)9/h10-18,22-25,28-31,44H,19-21H2,1-9H3,(H,39,47)(H,40,48)(H,41,46)(H,42,45)(H,43,49)/t24-,25+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50400803
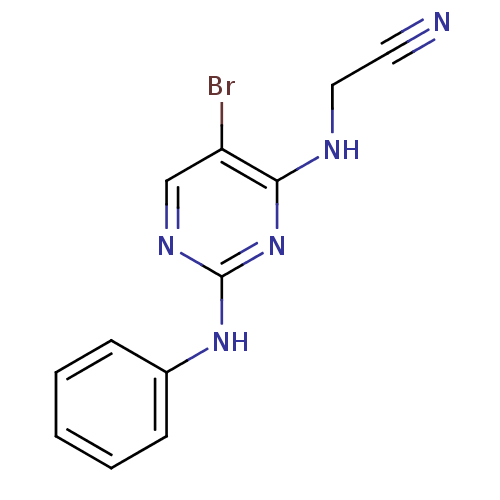
(CHEMBL2203628)Show InChI InChI=1S/C12H10BrN5/c13-10-8-16-12(18-11(10)15-7-6-14)17-9-4-2-1-3-5-9/h1-5,8H,7H2,(H2,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50400803

(CHEMBL2203628)Show InChI InChI=1S/C12H10BrN5/c13-10-8-16-12(18-11(10)15-7-6-14)17-9-4-2-1-3-5-9/h1-5,8H,7H2,(H2,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50400802

(CHEMBL1235529)Show InChI InChI=1S/C13H14BrN5O2S/c14-11-8-16-13-18-9-3-1-4-10(7-9)22(20,21)17-6-2-5-15-12(11)19-13/h1,3-4,7-8,17H,2,5-6H2,(H2,15,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50400799

(CHEMBL2203623)Show SMILES CC[C@H](C)[C@@H]1NCCOc2ccccc2CCCNC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CO)N(C)C1=O |r| Show InChI InChI=1S/C30H42N4O5/c1-4-21(2)27-30(38)34(3)25(20-35)29(37)33-24(19-22-11-6-5-7-12-22)28(36)32-16-10-14-23-13-8-9-15-26(23)39-18-17-31-27/h5-9,11-13,15,21,24-25,27,31,35H,4,10,14,16-20H2,1-3H3,(H,32,36)(H,33,37)/t21-,24+,25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Antagonist activity at ghrelin receptor assessed as inhibition of ghrelin-induced Ca2+ release by cell based assay |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003187
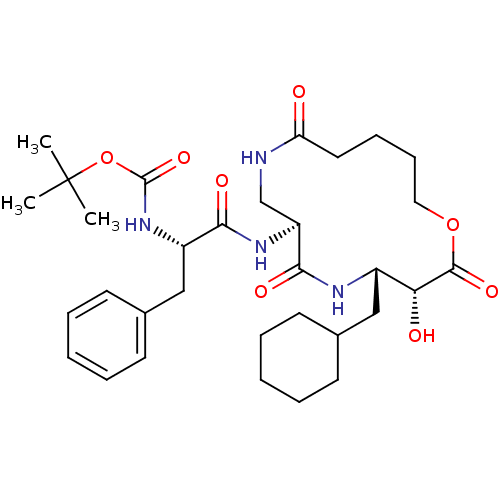
(CHEMBL92538 | [1-(4-Cyclohexylmethyl-3-hydroxy-2,6...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CNC(=O)CCCCOC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C32H48N4O8/c1-32(2,3)44-31(42)36-24(19-22-14-8-5-9-15-22)28(39)35-25-20-33-26(37)16-10-11-17-43-30(41)27(38)23(34-29(25)40)18-21-12-6-4-7-13-21/h5,8-9,14-15,21,23-25,27,38H,4,6-7,10-13,16-20H2,1-3H3,(H,33,37)(H,34,40)(H,35,39)(H,36,42)/t23-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50139762
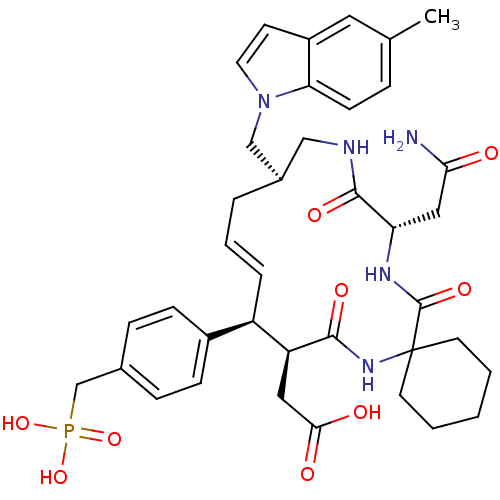
(CHEMBL349373 | [(16R,20S)-18-((S)-Carbamoylmethyl)...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(CP(O)(O)=O)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C38H48N5O9P/c1-24-8-13-32-28(18-24)14-17-43(32)22-26-6-5-7-29(27-11-9-25(10-12-27)23-53(50,51)52)30(19-34(45)46)35(47)42-38(15-3-2-4-16-38)37(49)41-31(20-33(39)44)36(48)40-21-26/h5,7-14,17-18,26,29-31H,2-4,6,15-16,19-23H2,1H3,(H2,39,44)(H,40,48)(H,41,49)(H,42,47)(H,45,46)(H2,50,51,52)/b7-5+/t26-,29-,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of SH2 domain of GRB2 in human MDA-MB-453 cells |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50400797

(CHEMBL2203622)Show InChI InChI=1S/C18H22N2O2/c1-20-16-14-19-18(20)22-13-9-5-3-2-4-8-12-21-17-11-7-6-10-15(16)17/h3,5-7,10-11,14H,2,4,8-9,12-13H2,1H3/b5-3- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A3 receptor |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50400797

(CHEMBL2203622)Show InChI InChI=1S/C18H22N2O2/c1-20-16-14-19-18(20)22-13-9-5-3-2-4-8-12-21-17-11-7-6-10-15(16)17/h3,5-7,10-11,14H,2,4,8-9,12-13H2,1H3/b5-3- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Antagonist activity at dopamine D1 receptor |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM15799

(CHEMBL81671 | Macrocyclic BACE inhibitor 1 | tert-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccncc1 |r| Show InChI InChI=1S/C37H56N6O7/c1-22(2)30(34(47)39-21-27-15-17-38-18-16-27)42-32(45)24(5)19-29(44)28(20-26-13-11-10-12-14-26)41-33(46)25(6)40-35(48)31(23(3)4)43-36(49)50-37(7,8)9/h10-18,22-25,28-31,44H,19-21H2,1-9H3,(H,39,47)(H,40,48)(H,41,46)(H,42,45)(H,43,49)/t24-,25+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by cell based assay |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50400810
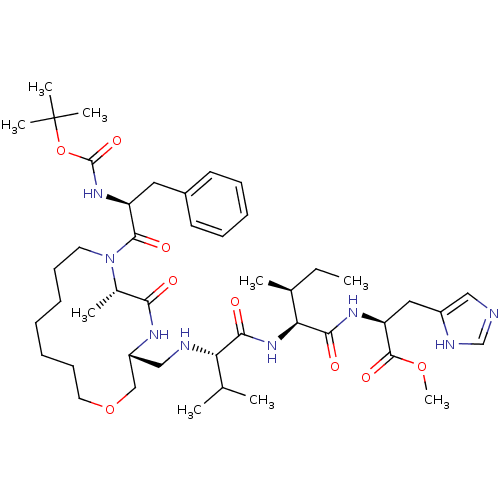
(CHEMBL2204330)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@@H]1COCCCCCCCN([C@@H](C)C(=O)N1)C(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)OC |r| Show InChI InChI=1S/C45H72N8O9/c1-10-30(4)38(41(56)50-36(43(58)60-9)24-33-25-46-28-48-33)52-40(55)37(29(2)3)47-26-34-27-61-22-18-13-11-12-17-21-53(31(5)39(54)49-34)42(57)35(23-32-19-15-14-16-20-32)51-44(59)62-45(6,7)8/h14-16,19-20,25,28-31,34-38,47H,10-13,17-18,21-24,26-27H2,1-9H3,(H,46,48)(H,49,54)(H,50,56)(H,51,59)(H,52,55)/t30-,31-,34+,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
High affinity choline transporter 1
(Homo sapiens (Human)) | BDBM50400797

(CHEMBL2203622)Show InChI InChI=1S/C18H22N2O2/c1-20-16-14-19-18(20)22-13-9-5-3-2-4-8-12-21-17-11-7-6-10-15(16)17/h3,5-7,10-11,14H,2,4,8-9,12-13H2,1H3/b5-3- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Antagonist activity at CHT1 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50400798
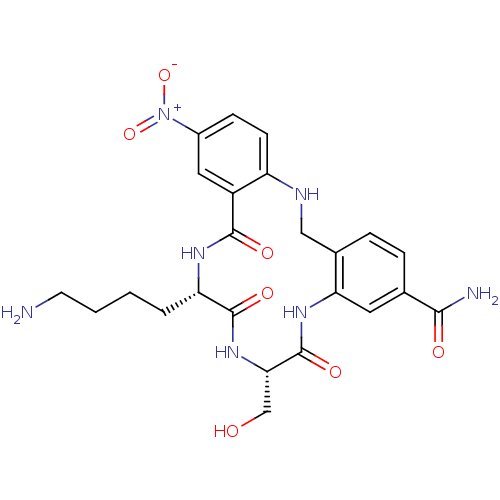
(CHEMBL336015)Show SMILES NCCCC[C@@H]1NC(=O)c2cc(ccc2NCc2ccc(cc2NC(=O)[C@H](CO)NC1=O)C(N)=O)[N+]([O-])=O Show InChI InChI=1S/C24H29N7O7/c25-8-2-1-3-18-23(35)30-20(12-32)24(36)29-19-9-13(21(26)33)4-5-14(19)11-27-17-7-6-15(31(37)38)10-16(17)22(34)28-18/h4-7,9-10,18,20,27,32H,1-3,8,11-12,25H2,(H2,26,33)(H,28,34)(H,29,36)(H,30,35)/t18-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of TrkA assessed as inhibition of mAb5C3 binding |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50056251

((6R,9S)-6-Mercaptomethyl-7-oxo-5,6,7,8,9,10,11,12,...)Show SMILES OC(=O)[C@@H]1CCCCCc2ccccc2C[C@@H](CS)C(=O)N1 Show InChI InChI=1S/C17H23NO3S/c19-16-14(11-22)10-13-8-5-4-7-12(13)6-2-1-3-9-15(18-16)17(20)21/h4-5,7-8,14-15,22H,1-3,6,9-11H2,(H,18,19)(H,20,21)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50139762

(CHEMBL349373 | [(16R,20S)-18-((S)-Carbamoylmethyl)...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(CP(O)(O)=O)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C38H48N5O9P/c1-24-8-13-32-28(18-24)14-17-43(32)22-26-6-5-7-29(27-11-9-25(10-12-27)23-53(50,51)52)30(19-34(45)46)35(47)42-38(15-3-2-4-16-38)37(49)41-31(20-33(39)44)36(48)40-21-26/h5,7-14,17-18,26,29-31H,2-4,6,15-16,19-23H2,1H3,(H2,39,44)(H,40,48)(H,41,49)(H,42,47)(H,45,46)(H2,50,51,52)/b7-5+/t26-,29-,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of SH2 domain of GRB2 in human MDA-MB-231 cells |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50400809
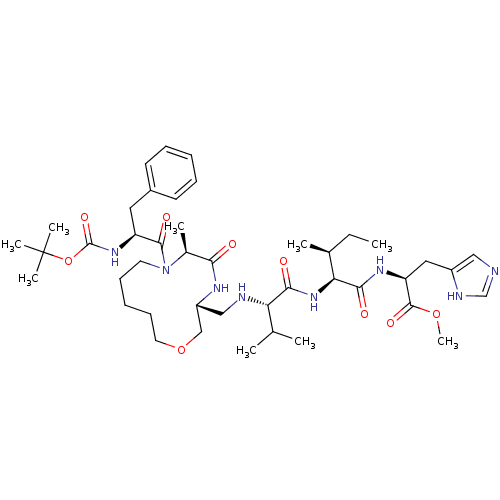
(CHEMBL2204331)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@@H]1COCCCCCN([C@@H](C)C(=O)N1)C(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)OC |r| Show InChI InChI=1S/C43H68N8O9/c1-10-28(4)36(39(54)48-34(41(56)58-9)22-31-23-44-26-46-31)50-38(53)35(27(2)3)45-24-32-25-59-20-16-12-15-19-51(29(5)37(52)47-32)40(55)33(21-30-17-13-11-14-18-30)49-42(57)60-43(6,7)8/h11,13-14,17-18,23,26-29,32-36,45H,10,12,15-16,19-22,24-25H2,1-9H3,(H,44,46)(H,47,52)(H,48,54)(H,49,57)(H,50,53)/t28-,29-,32+,33-,34-,35-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50400814

(CHEMBL2204332)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@@H]1COCCCN([C@@H](C)C(=O)N1)C(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)OC |r| Show InChI InChI=1S/C41H64N8O9/c1-10-26(4)34(37(52)46-32(39(54)56-9)20-29-21-42-24-44-29)48-36(51)33(25(2)3)43-22-30-23-57-18-14-17-49(27(5)35(50)45-30)38(53)31(19-28-15-12-11-13-16-28)47-40(55)58-41(6,7)8/h11-13,15-16,21,24-27,30-34,43H,10,14,17-20,22-23H2,1-9H3,(H,42,44)(H,45,50)(H,46,52)(H,47,55)(H,48,51)/t26-,27-,30+,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of renin |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50359255
(CHEMBL1923501)Show SMILES CCC[C@@H]1NCCOc2ccccc2\C=C\CNC(=O)[C@@H](Cc2ccccc2)NC(=O)CN(C)C1=O |r,t:15| Show InChI InChI=1S/C28H36N4O4/c1-3-10-23-28(35)32(2)20-26(33)31-24(19-21-11-5-4-6-12-21)27(34)30-16-9-14-22-13-7-8-15-25(22)36-18-17-29-23/h4-9,11-15,23-24,29H,3,10,16-20H2,1-2H3,(H,30,34)(H,31,33)/b14-9+/t23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 68 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Agonist activity at ghrelin receptor by cell based assay |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50359256
(CHEMBL1923502 | ULIMORELIN)Show SMILES C[C@@H]1CN[C@@H](C2CC2)C(=O)N(C)[C@H](C)C(=O)N[C@H](Cc2ccc(F)cc2)C(=O)NCCCc2ccccc2O1 |r| Show InChI InChI=1S/C30H39FN4O4/c1-19-18-33-27(23-12-13-23)30(38)35(3)20(2)28(36)34-25(17-21-10-14-24(31)15-11-21)29(37)32-16-6-8-22-7-4-5-9-26(22)39-19/h4-5,7,9-11,14-15,19-20,23,25,27,33H,6,8,12-13,16-18H2,1-3H3,(H,32,37)(H,34,36)/t19-,20-,25-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Agonist activity at ghrelin receptor by cell based assay |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Sonic hedgehog protein
(Mus musculus (Mouse)) | BDBM50249181

(CHEMBL473392 | N-(4-chlorobenzyl)-2-((2R,6S,E)-5,1...)Show SMILES Clc1ccc(CNC(=O)C[C@@H]2C\C=C\CCC(=O)O[C@@H](CNC2=O)c2ccccc2)cc1 |r,t:12| Show InChI InChI=1S/C25H27ClN2O4/c26-21-13-11-18(12-14-21)16-27-23(29)15-20-9-5-2-6-10-24(30)32-22(17-28-25(20)31)19-7-3-1-4-8-19/h1-5,7-8,11-14,20,22H,6,9-10,15-17H2,(H,27,29)(H,28,31)/b5-2+/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of sonic hedgehog pathway in mouse NIH-3T3 cells |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
DnaJ homolog subfamily A member 1
(Homo sapiens (Human)) | BDBM50139187
(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of HDJ2 |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |
Ras-related protein Rap-1A
(Homo sapiens (Human)) | BDBM50139187

(4-(2-cyclohexylethyl)-23-oxo-8-oxa-1,15,17,21-tetr...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CCC1CCCCC1 Show InChI InChI=1S/C31H35N5O2/c32-16-26-9-7-24-14-30(26)38-29-11-10-25(8-6-23-4-2-1-3-5-23)27(15-29)19-35-13-12-34(21-31(35)37)20-28-17-33-22-36(28)18-24/h7,9-11,14-15,17,22-23H,1-6,8,12-13,18-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Inhibition of Rap1a |
J Med Chem 54: 1961-2004 (2011)
Article DOI: 10.1021/jm1012374
BindingDB Entry DOI: 10.7270/Q28C9XDV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data