

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22915 (3-(1H-imidazol-4-yl)propylphenylmethyl ether | 5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403581 (CHEMBL291369) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403577 (CHEMBL443322) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403575 (CHEMBL59679) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403578 (CHEMBL60342) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403580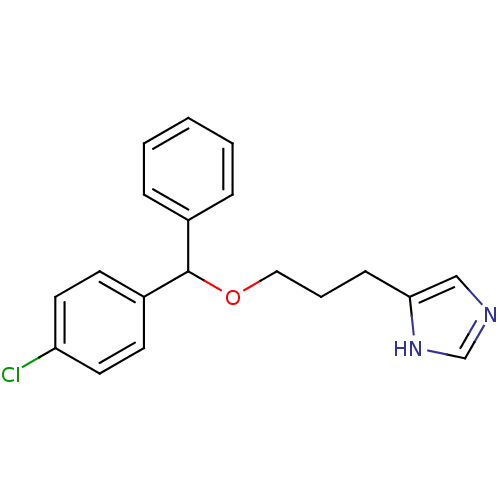 (CHEMBL62244) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403576 (CHEMBL59851) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50403582 (CHEMBL60606) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||