 Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50029866
Found 33 hits Enz. Inhib. hit(s) with all data for entry = 50029866 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039257
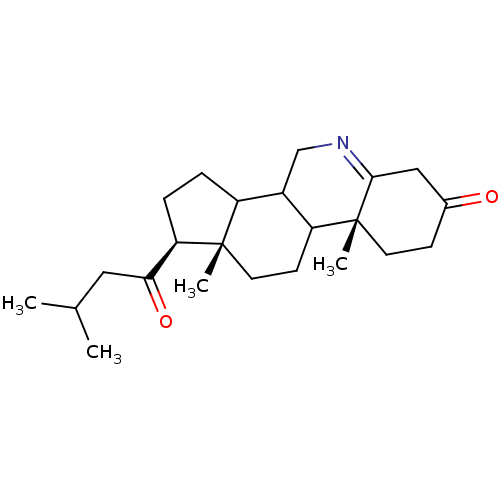
((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604
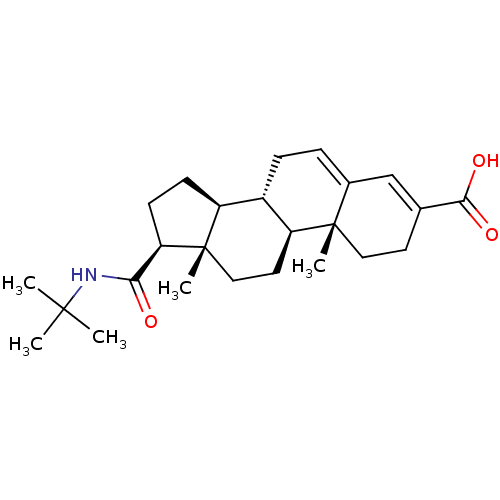
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403606
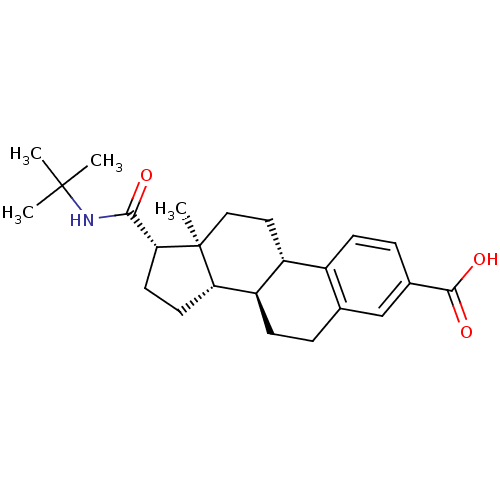
(CHEMBL1627951)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C24H33NO3/c1-23(2,3)25-21(26)20-10-9-19-18-8-5-14-13-15(22(27)28)6-7-16(14)17(18)11-12-24(19,20)4/h6-7,13,17-20H,5,8-12H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039285
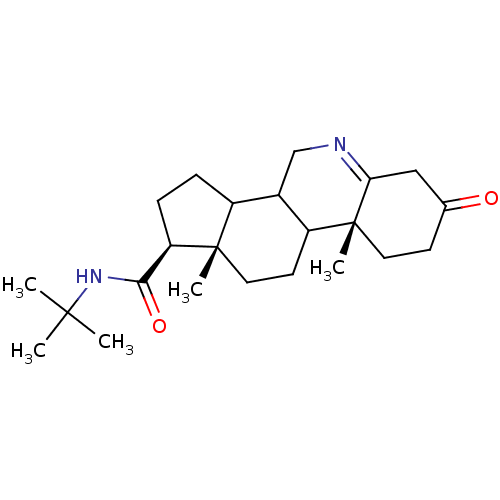
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)18-7-6-16-15-13-24-19-12-14(26)8-10-23(19,5)17(15)9-11-22(16,18)4/h15-18H,6-13H2,1-5H3,(H,25,27)/t15?,16?,17?,18-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403610
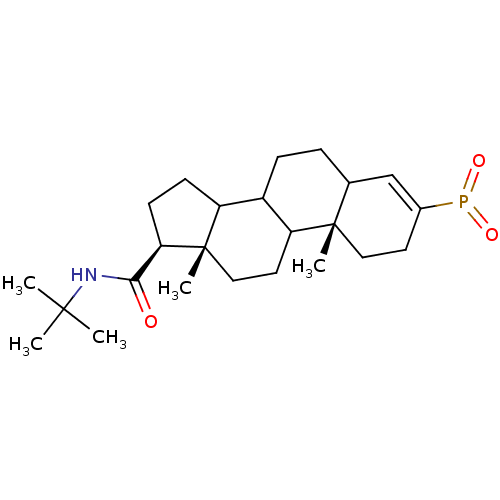
(CHEMBL143220)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CCC4C=C(CC[C@]4(C)C3CC[C@]12C)P(=O)=O |c:15| Show InChI InChI=1S/C24H38NO3P/c1-22(2,3)25-21(26)20-9-8-18-17-7-6-15-14-16(29(27)28)10-12-23(15,4)19(17)11-13-24(18,20)5/h14-15,17-20H,6-13H2,1-5H3,(H,25,26)/t15?,17?,18?,19?,20-,23+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50213061
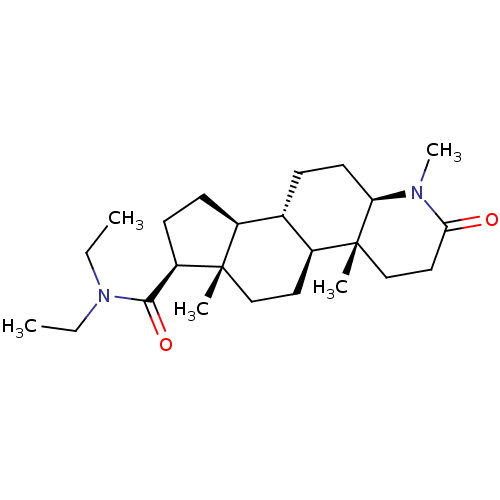
(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50213061

(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50039257

((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057479
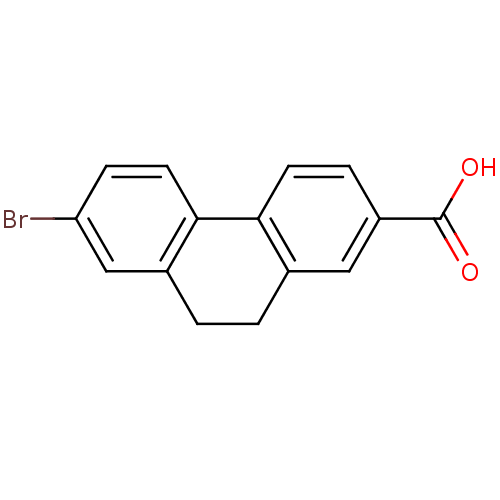
(7-Bromo-9,10-dihydro-phenanthrene-2-carboxylic aci...)Show InChI InChI=1S/C15H11BrO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391268

(CHEMBL110001)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)[N+]([O-])=O |c:17| Show InChI InChI=1S/C26H42N2O3/c1-16(2)27(17(3)4)24(29)23-10-9-21-20-8-7-18-15-19(28(30)31)11-13-25(18,5)22(20)12-14-26(21,23)6/h15-18,20-23H,7-14H2,1-6H3/t18-,20?,21?,22?,23+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057501
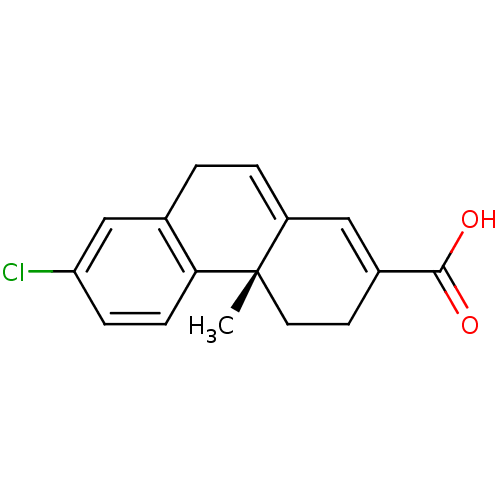
((S)-7-Chloro-4a-methyl-3,4,4a,9-tetrahydro-phenant...)Show SMILES C[C@]12CCC(=CC1=CCc1cc(Cl)ccc21)C(O)=O |c:4,7| Show InChI InChI=1S/C16H15ClO2/c1-16-7-6-11(15(18)19)8-12(16)3-2-10-9-13(17)4-5-14(10)16/h3-5,8-9H,2,6-7H2,1H3,(H,18,19)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403607
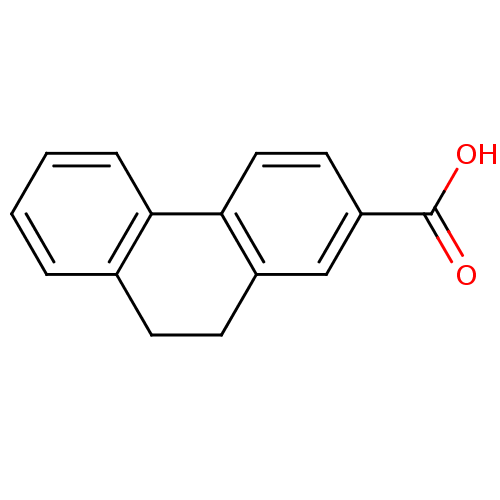
(CHEMBL341625)Show InChI InChI=1S/C15H12O2/c16-15(17)12-7-8-14-11(9-12)6-5-10-3-1-2-4-13(10)14/h1-4,7-9H,5-6H2,(H,16,17) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
| 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403611
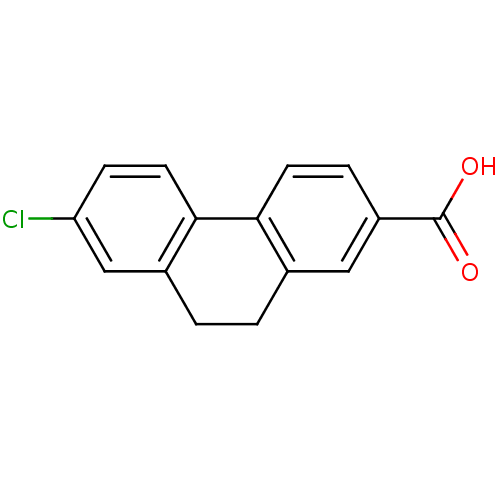
(CHEMBL356780)Show InChI InChI=1S/C15H11ClO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50039285

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)18-7-6-16-15-13-24-19-12-14(26)8-10-23(19,5)17(15)9-11-22(16,18)4/h15-18H,6-13H2,1-5H3,(H,25,27)/t15?,16?,17?,18-,22+,23-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50072190
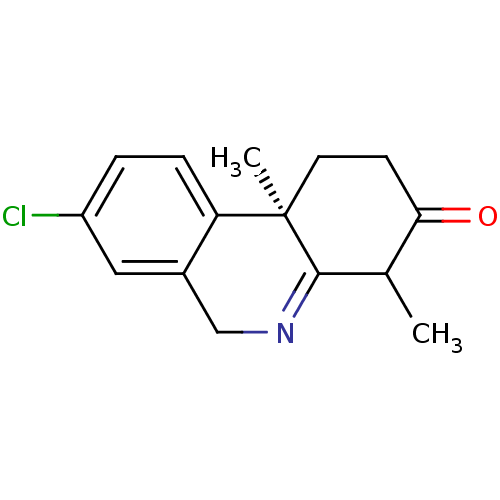
((R)-8-Chloro-4,10b-dimethyl-1,5,6,10b-tetrahydro-2...)Show SMILES CC1C(=O)CC[C@@]2(C)C1=NCc1cc(Cl)ccc21 |t:9| Show InChI InChI=1S/C15H16ClNO/c1-9-13(18)5-6-15(2)12-4-3-11(16)7-10(12)8-17-14(9)15/h3-4,7,9H,5-6,8H2,1-2H3/t9?,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057501

((S)-7-Chloro-4a-methyl-3,4,4a,9-tetrahydro-phenant...)Show SMILES C[C@]12CCC(=CC1=CCc1cc(Cl)ccc21)C(O)=O |c:4,7| Show InChI InChI=1S/C16H15ClO2/c1-16-7-6-11(15(18)19)8-12(16)3-2-10-9-13(17)4-5-14(10)16/h3-5,8-9H,2,6-7H2,1H3,(H,18,19)/t16-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403606

(CHEMBL1627951)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C24H33NO3/c1-23(2,3)25-21(26)20-10-9-19-18-8-5-14-13-15(22(27)28)6-7-16(14)17(18)11-12-24(19,20)4/h6-7,13,17-20H,5,8-12H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-,19+,20-,24+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403608
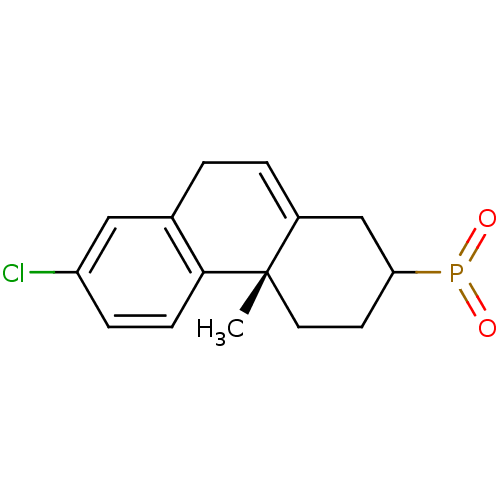
(CHEMBL439804)Show SMILES C[C@]12CCC(CC1=CCc1cc(Cl)ccc21)P(=O)=O |c:7| Show InChI InChI=1S/C15H16ClO2P/c1-15-7-6-13(19(17)18)9-11(15)3-2-10-8-12(16)4-5-14(10)15/h3-5,8,13H,2,6-7,9H2,1H3/t13?,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403608

(CHEMBL439804)Show SMILES C[C@]12CCC(CC1=CCc1cc(Cl)ccc21)P(=O)=O |c:7| Show InChI InChI=1S/C15H16ClO2P/c1-15-7-6-13(19(17)18)9-11(15)3-2-10-8-12(16)4-5-14(10)15/h3-5,8,13H,2,6-7,9H2,1H3/t13?,15-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403605
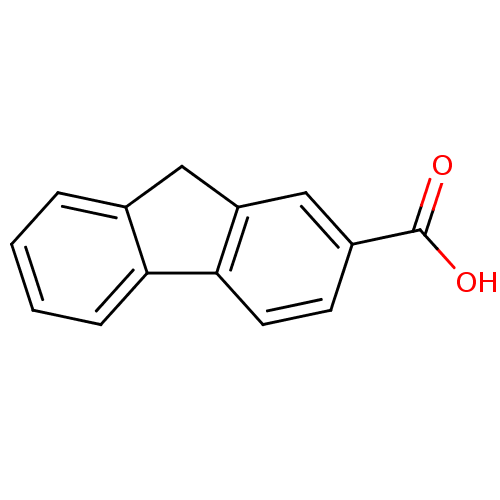
(CHEMBL144346)Show InChI InChI=1S/C14H10O2/c15-14(16)10-5-6-13-11(8-10)7-9-3-1-2-4-12(9)13/h1-6,8H,7H2,(H,15,16) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403609
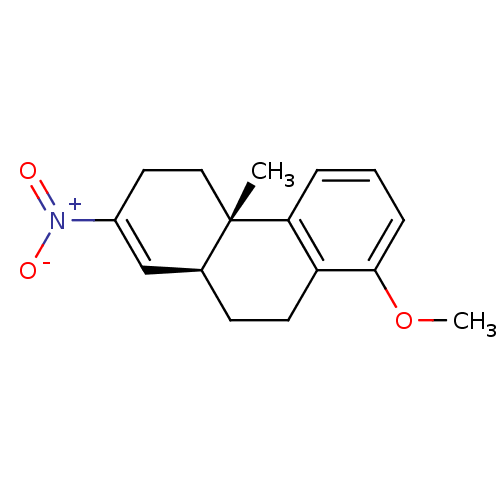
(CHEMBL341666)Show SMILES COc1cccc2c1CC[C@H]1C=C(CC[C@]21C)[N+]([O-])=O |c:12| Show InChI InChI=1S/C16H19NO3/c1-16-9-8-12(17(18)19)10-11(16)6-7-13-14(16)4-3-5-15(13)20-2/h3-5,10-11H,6-9H2,1-2H3/t11-,16-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403611

(CHEMBL356780)Show InChI InChI=1S/C15H11ClO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50403610

(CHEMBL143220)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CCC4C=C(CC[C@]4(C)C3CC[C@]12C)P(=O)=O |c:15| Show InChI InChI=1S/C24H38NO3P/c1-22(2,3)25-21(26)20-9-8-18-17-7-6-15-14-16(29(27)28)10-12-23(15,4)19(17)11-13-24(18,20)5/h14-15,17-20H,6-13H2,1-5H3,(H,25,26)/t15?,17?,18?,19?,20-,23+,24+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50391268

(CHEMBL110001)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)[N+]([O-])=O |c:17| Show InChI InChI=1S/C26H42N2O3/c1-16(2)27(17(3)4)24(29)23-10-9-21-20-8-7-18-15-19(28(30)31)11-13-25(18,5)22(20)12-14-26(21,23)6/h15-18,20-23H,7-14H2,1-6H3/t18-,20?,21?,22?,23+,25-,26-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057479

(7-Bromo-9,10-dihydro-phenanthrene-2-carboxylic aci...)Show InChI InChI=1S/C15H11BrO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403605

(CHEMBL144346)Show InChI InChI=1S/C14H10O2/c15-14(16)10-5-6-13-11(8-10)7-9-3-1-2-4-12(9)13/h1-6,8H,7H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403607
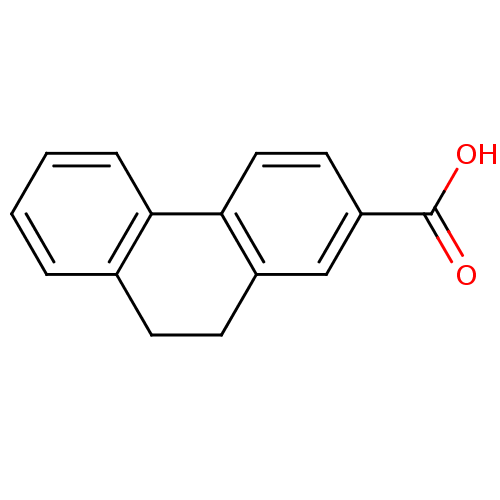
(CHEMBL341625)Show InChI InChI=1S/C15H12O2/c16-15(17)12-7-8-14-11(9-12)6-5-10-3-1-2-4-13(10)14/h1-4,7-9H,5-6H2,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50072190

((R)-8-Chloro-4,10b-dimethyl-1,5,6,10b-tetrahydro-2...)Show SMILES CC1C(=O)CC[C@@]2(C)C1=NCc1cc(Cl)ccc21 |t:9| Show InChI InChI=1S/C15H16ClNO/c1-9-13(18)5-6-15(2)12-4-3-11(16)7-10(12)8-17-14(9)15/h3-4,7,9H,5-6,8H2,1-2H3/t9?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50025356
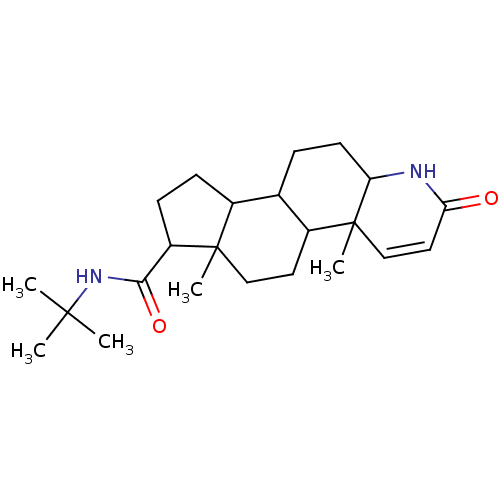
(4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10...)Show SMILES CC(C)(C)NC(=O)C1CCC2C3CCC4NC(=O)C=CC4(C)C3CCC12C |c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50025356

(4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10...)Show SMILES CC(C)(C)NC(=O)C1CCC2C3CCC4NC(=O)C=CC4(C)C3CCC12C |c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against type-1 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data