 Found 180 hits Enz. Inhib. hit(s) with all data for entry = 50041259
Found 180 hits Enz. Inhib. hit(s) with all data for entry = 50041259 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50408384

(CHEMBL136526)Show SMILES CNC(=O)Oc1ccc2N(C)C3[C@@](C)(CC[N+]3(C)C)c2c1 Show InChI InChI=1S/C16H23N3O2/c1-16-8-9-19(4,5)14(16)18(3)13-7-6-11(10-12(13)16)21-15(20)17-2/h6-7,10,14H,8-9H2,1-5H3/p+1/t14?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 57.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50014978
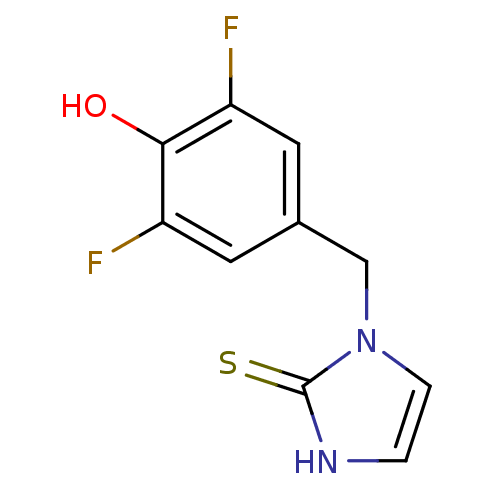
(1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...)Show InChI InChI=1S/C10H8F2N2OS/c11-7-3-6(4-8(12)9(7)15)5-14-2-1-13-10(14)16/h1-4,15H,5H2,(H,13,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 76.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 91.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014109

((-)-Octyl-carbamic acid 1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCCCCCNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C22H35N3O2/c1-5-6-7-8-9-10-14-23-21(26)27-17-11-12-19-18(16-17)22(2)13-15-24(3)20(22)25(19)4/h11-12,16,20H,5-10,13-15H2,1-4H3,(H,23,26)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055207

((S)-5-Butylcarbamoyloxy-1,3a,8-trimethyl-1,2,3,3a,...)Show SMILES CCCCNC(=O)Oc1ccc2N(C)C3[N@H+](C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C18H27N3O2/c1-5-6-10-19-17(22)23-13-7-8-15-14(12-13)18(2)9-11-20(3)16(18)21(15)4/h7-8,12,16H,5-6,9-11H2,1-4H3,(H,19,22)/p+1/t16?,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10622
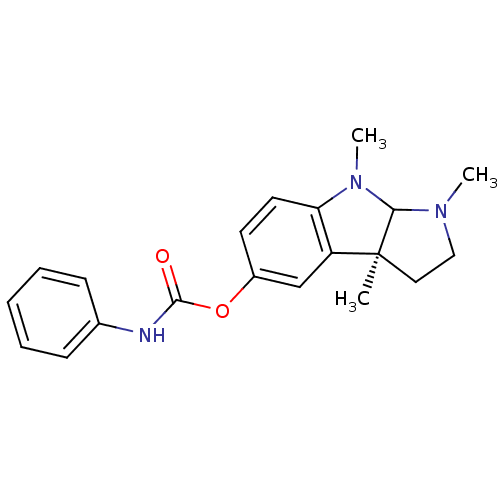
((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...)Show SMILES CN1CC[C@]2(C)C1N(C)c1ccc(OC(=O)Nc3ccccc3)cc21 |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055156
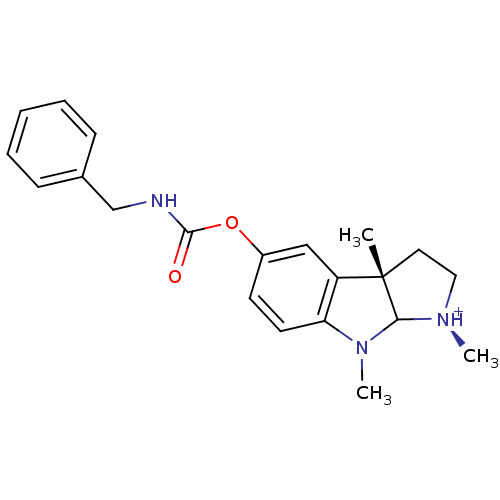
((S)-5-Benzylcarbamoyloxy-1,3a,8-trimethyl-1,2,3,3a...)Show SMILES CN1C2[N@H+](C)CC[C@@]2(C)c2cc(OC(=O)NCc3ccccc3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)26-20(25)22-14-15-7-5-4-6-8-15/h4-10,13,19H,11-12,14H2,1-3H3,(H,22,25)/p+1/t19?,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055192
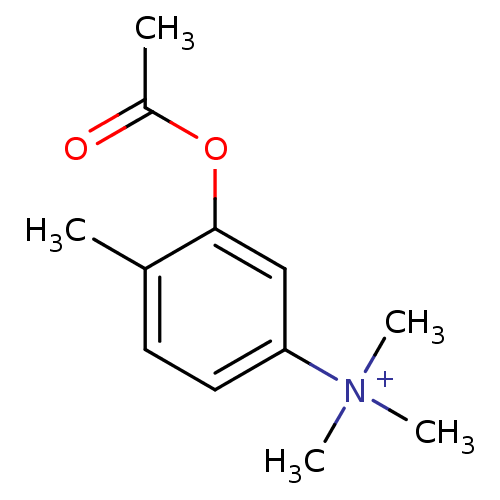
((3-Acetoxy-4-methyl-phenyl)-trimethyl-ammonium | C...)Show InChI InChI=1S/C12H18NO2/c1-9-6-7-11(13(3,4)5)8-12(9)15-10(2)14/h6-8H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 659 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025811
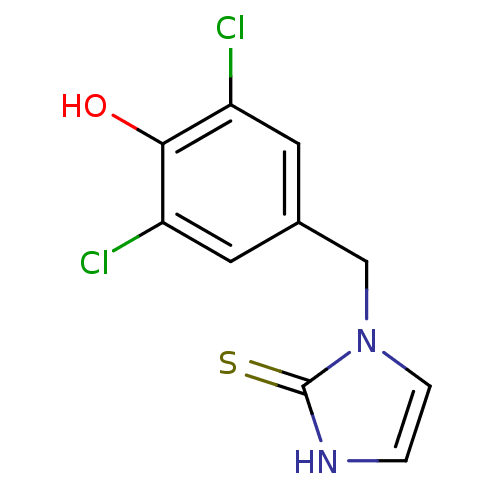
(1-(3,5-Dichloro-4-hydroxy-benzyl)-1,3-dihydro-imid...)Show InChI InChI=1S/C10H8Cl2N2OS/c11-7-3-6(4-8(12)9(7)15)5-14-2-1-13-10(14)16/h1-4,15H,5H2,(H,13,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262
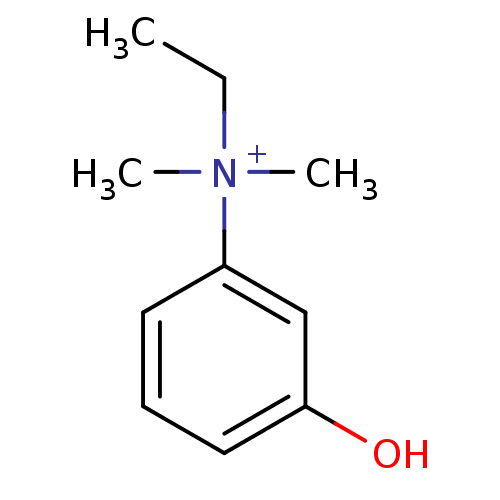
(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10984
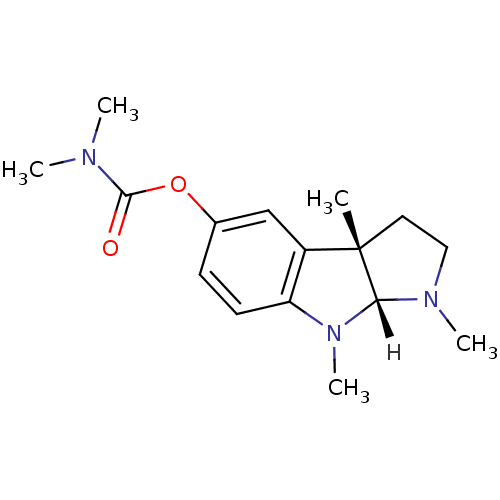
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)C)ccc1N2C |r| Show InChI InChI=1S/C16H23N3O2/c1-16-8-9-18(4)14(16)19(5)13-7-6-11(10-12(13)16)21-15(20)17(2)3/h6-7,10,14H,8-9H2,1-5H3/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 971 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055186

((3-Hydroxy-phenyl)-trimethyl-ammonium | CHEMBL3777...)Show InChI InChI=1S/C9H13NO/c1-10(2,3)8-5-4-6-9(11)7-8/h4-7H,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 973 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50014983
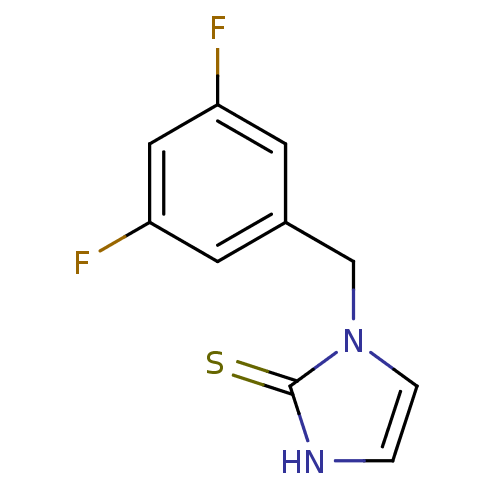
(1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...)Show InChI InChI=1S/C10H8F2N2S/c11-8-3-7(4-9(12)5-8)6-14-2-1-13-10(14)15/h1-5H,6H2,(H,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025795
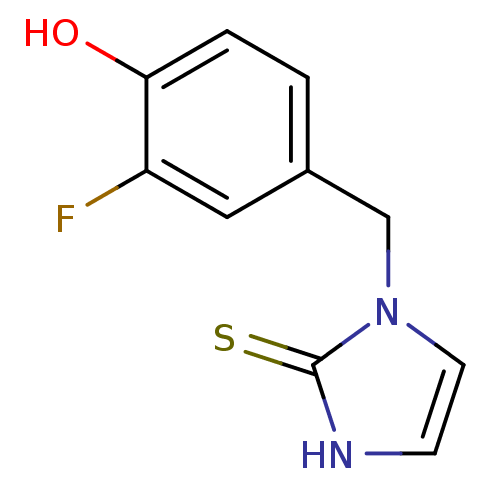
(1-(3-Fluoro-4-hydroxy-benzyl)-1,3-dihydro-imidazol...)Show InChI InChI=1S/C10H9FN2OS/c11-8-5-7(1-2-9(8)14)6-13-4-3-12-10(13)15/h1-5,14H,6H2,(H,12,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50014107
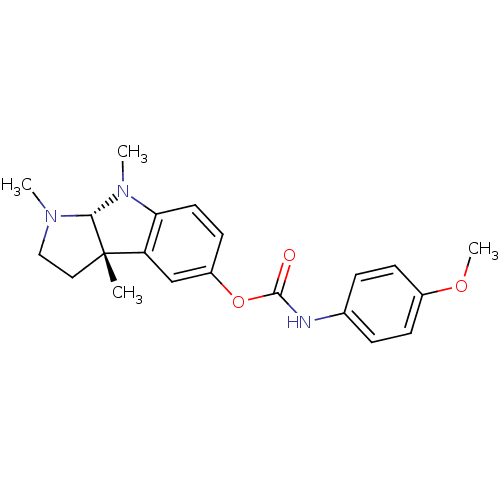
((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055181
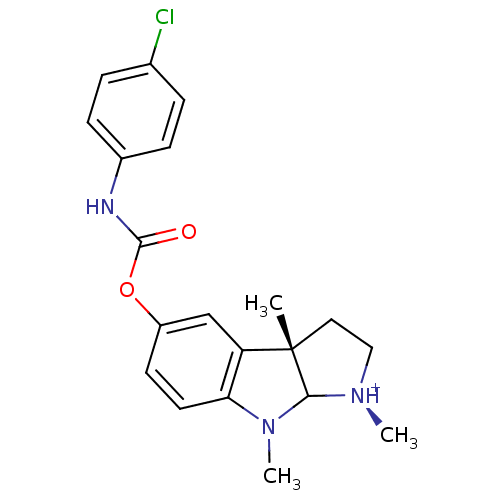
((S)-5-(4-Chloro-phenylcarbamoyloxy)-1,3a,8-trimeth...)Show SMILES CN1C2[N@H+](C)CC[C@@]2(C)c2cc(OC(=O)Nc3ccc(Cl)cc3)ccc12 Show InChI InChI=1S/C20H22ClN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-15(12-16(17)20)26-19(25)22-14-6-4-13(21)5-7-14/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/p+1/t18?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025806
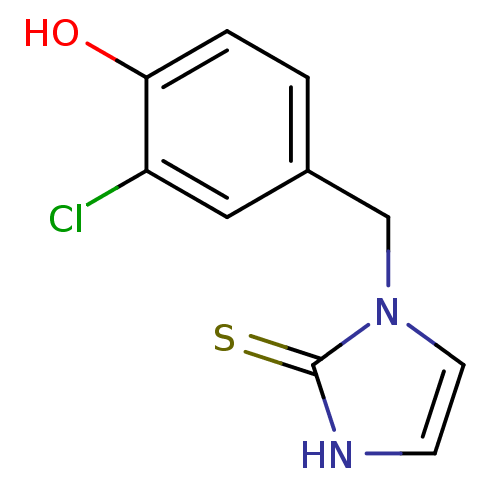
(1-(3-Chloro-4-hydroxy-benzyl)-1,3-dihydro-imidazol...)Show InChI InChI=1S/C10H9ClN2OS/c11-8-5-7(1-2-9(8)14)6-13-4-3-12-10(13)15/h1-5,14H,6H2,(H,12,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025803

(1-(3,4-Dihydroxy-benzyl)-1,3-dihydro-imidazole-2-t...)Show InChI InChI=1S/C10H10N2O2S/c13-8-2-1-7(5-9(8)14)6-12-4-3-11-10(12)15/h1-5,13-14H,6H2,(H,11,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025793

(1-(3,5-Dichloro-benzyl)-1,3-dihydro-imidazole-2-th...)Show InChI InChI=1S/C10H8Cl2N2S/c11-8-3-7(4-9(12)5-8)6-14-2-1-13-10(14)15/h1-5H,6H2,(H,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50014968
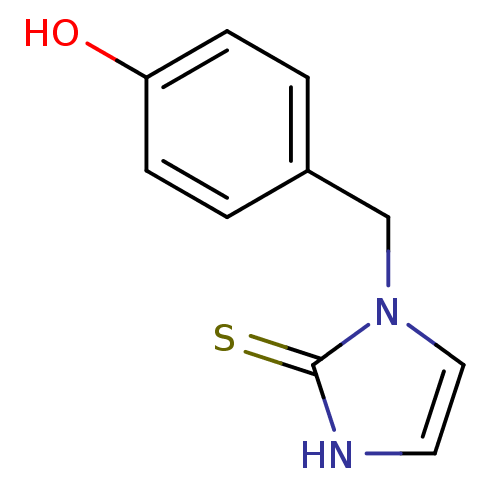
(1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...)Show InChI InChI=1S/C10H10N2OS/c13-9-3-1-8(2-4-9)7-12-6-5-11-10(12)14/h1-6,13H,7H2,(H,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055185

((5-Hydroxy-2-methyl-phenyl)-trimethyl-ammonium | C...)Show InChI InChI=1S/C10H15NO/c1-8-5-6-9(12)7-10(8)11(2,3)4/h5-7H,1-4H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055199

((3-Acetoxy-phenyl)-trimethyl-ammonium | CHEMBL3433...)Show InChI InChI=1S/C11H16NO2/c1-9(13)14-11-7-5-6-10(8-11)12(2,3)4/h5-8H,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055204

((3-Hydroxy-4-methyl-phenyl)-trimethyl-ammonium | C...)Show InChI InChI=1S/C10H15NO/c1-8-5-6-9(7-10(8)12)11(2,3)4/h5-7H,1-4H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055193
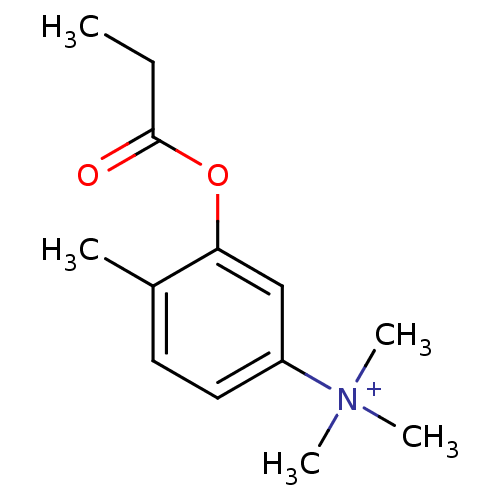
(CHEMBL138072 | Trimethyl-(4-methyl-3-propionyloxy-...)Show InChI InChI=1S/C13H20NO2/c1-6-13(15)16-12-9-11(14(3,4)5)8-7-10(12)2/h7-9H,6H2,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055188
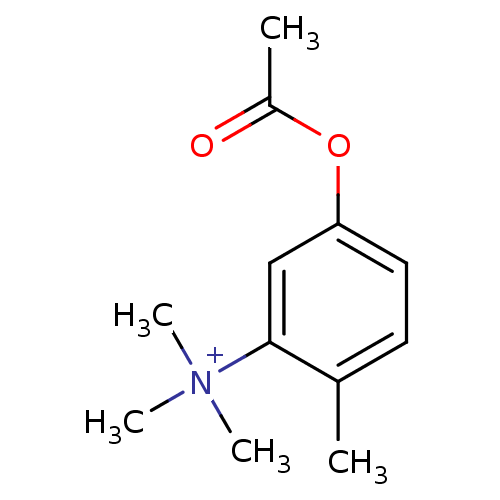
((5-Acetoxy-2-methyl-phenyl)-trimethyl-ammonium | C...)Show InChI InChI=1S/C12H18NO2/c1-9-6-7-11(15-10(2)14)8-12(9)13(3,4)5/h6-8H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50014969
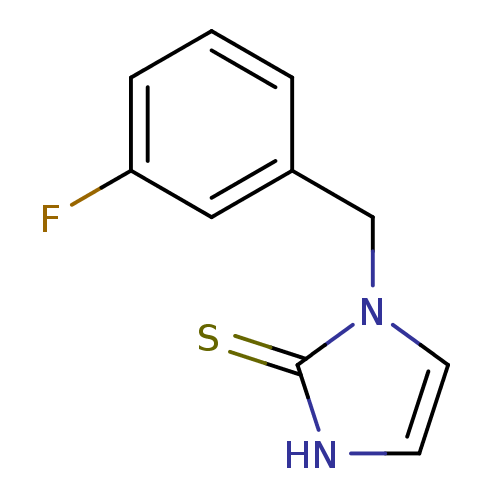
(1-(3-Fluoro-benzyl)-1,3-dihydro-imidazole-2-thione...)Show InChI InChI=1S/C10H9FN2S/c11-9-3-1-2-8(6-9)7-13-5-4-12-10(13)14/h1-6H,7H2,(H,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055157

(CHEMBL136294 | Trimethyl-(3-propionyloxy-phenyl)-a...)Show InChI InChI=1S/C12H18NO2/c1-5-12(14)15-11-8-6-7-10(9-11)13(2,3)4/h6-9H,5H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055178
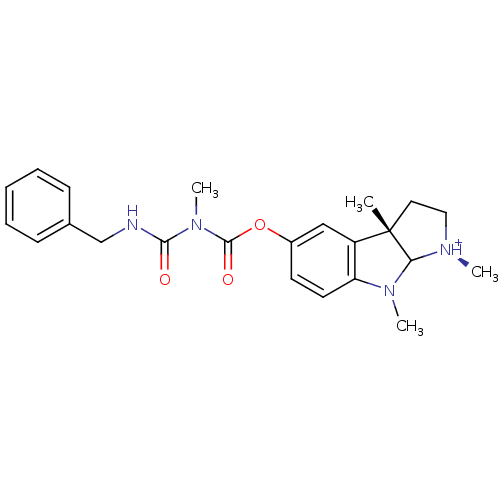
(5-(2-Benzyl-acetamino-methylcarbomylo)-1,3a,8-trim...)Show SMILES CN(C(=O)NCc1ccccc1)C(=O)Oc1ccc2N(C)C3[N@H+](C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C23H28N4O3/c1-23-12-13-25(2)20(23)26(3)19-11-10-17(14-18(19)23)30-22(29)27(4)21(28)24-15-16-8-6-5-7-9-16/h5-11,14,20H,12-13,15H2,1-4H3,(H,24,28)/p+1/t20?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025810
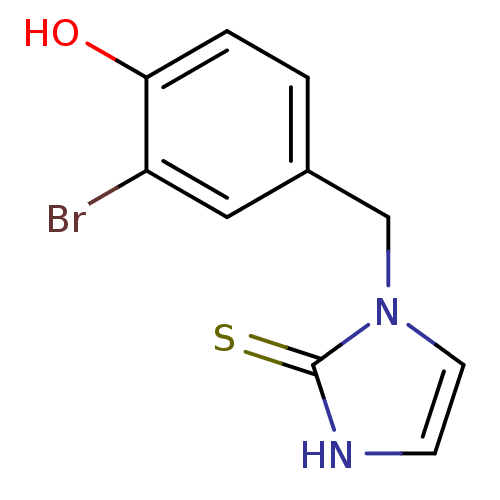
(1-(3-Bromo-4-hydroxy-benzyl)-1,3-dihydro-imidazole...)Show InChI InChI=1S/C10H9BrN2OS/c11-8-5-7(1-2-9(8)14)6-13-4-3-12-10(13)15/h1-5,14H,6H2,(H,12,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
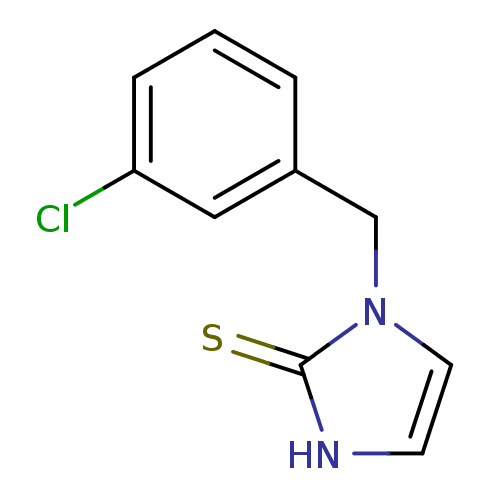
(Homo sapiens (Human)) | BDBM50025789
(1-(3-Chloro-benzyl)-1,3-dihydro-imidazole-2-thione...)Show InChI InChI=1S/C10H9ClN2S/c11-9-3-1-2-8(6-9)7-13-5-4-12-10(13)14/h1-6H,7H2,(H,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025791
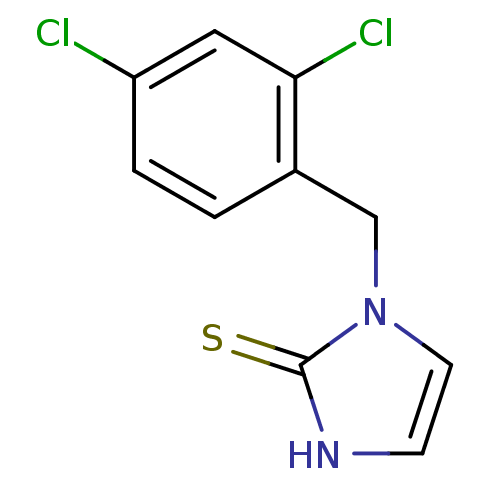
(1-(2,4-Dichloro-benzyl)-1,3-dihydro-imidazole-2-th...)Show InChI InChI=1S/C10H8Cl2N2S/c11-8-2-1-7(9(12)5-8)6-14-4-3-13-10(14)15/h1-5H,6H2,(H,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025797

(1-(3,4-Dichloro-benzyl)-1,3-dihydro-imidazole-2-th...)Show InChI InChI=1S/C10H8Cl2N2S/c11-8-2-1-7(5-9(8)12)6-14-4-3-13-10(14)15/h1-5H,6H2,(H,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025785

(1-(4-Hydroxy-3-nitro-benzyl)-1,3-dihydro-imidazole...)Show InChI InChI=1S/C10H9N3O3S/c14-9-2-1-7(5-8(9)13(15)16)6-12-4-3-11-10(12)17/h1-5,14H,6H2,(H,11,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50014960
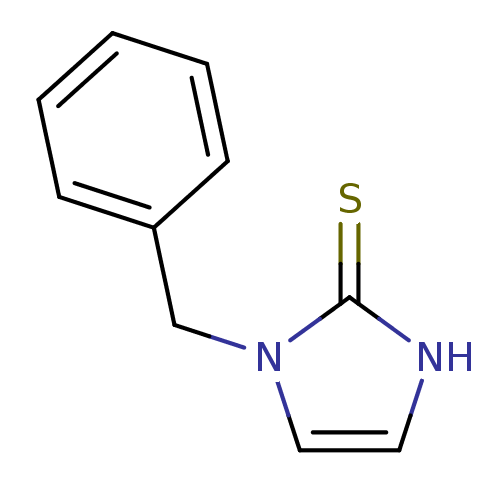
(1-Benzyl-1,3-dihydro-imidazole-2-thione | 1-Benzyl...)Show InChI InChI=1S/C10H10N2S/c13-10-11-6-7-12(10)8-9-4-2-1-3-5-9/h1-7H,8H2,(H,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055200

((S)-5-Isopropylcarbamoyloxy-1,3a,8-trimethyl-1,2,3...)Show SMILES CC(C)NC(=O)Oc1ccc2N(C)C3[N@H+](C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C17H25N3O2/c1-11(2)18-16(21)22-12-6-7-14-13(10-12)17(3)8-9-19(4)15(17)20(14)5/h6-7,10-11,15H,8-9H2,1-5H3,(H,18,21)/p+1/t15?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025790

(1-(3,5-Difluoro-4-methoxy-benzyl)-1,3-dihydro-imid...)Show InChI InChI=1S/C11H10F2N2OS/c1-16-10-8(12)4-7(5-9(10)13)6-15-3-2-14-11(15)17/h2-5H,6H2,1H3,(H,14,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055160

((S)-5-Diethylcarbamoyloxy-1,3a,8-trimethyl-1,2,3,3...)Show SMILES CCN(CC)C(=O)Oc1ccc2N(C)C3[N@H+](C)CC[C@@]3(C)c2c1 Show InChI InChI=1S/C18H27N3O2/c1-6-21(7-2)17(22)23-13-8-9-15-14(12-13)18(3)10-11-19(4)16(18)20(15)5/h8-9,12,16H,6-7,10-11H2,1-5H3/p+1/t16?,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055171

((3-Acetoxy-2-methyl-phenyl)-trimethyl-ammonium | C...)Show InChI InChI=1S/C12H18NO2/c1-9-11(13(3,4)5)7-6-8-12(9)15-10(2)14/h6-8H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025802
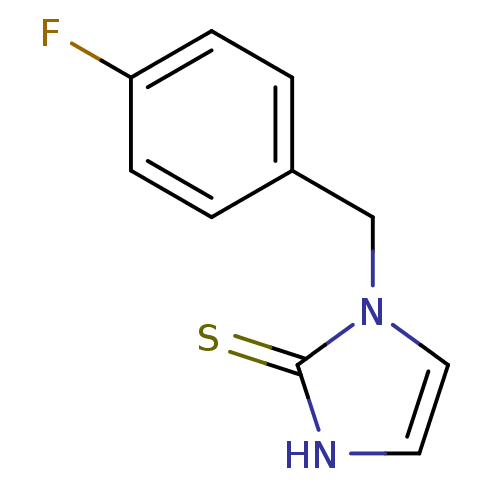
(1-(4-Fluoro-benzyl)-1,3-dihydro-imidazole-2-thione...)Show InChI InChI=1S/C10H9FN2S/c11-9-3-1-8(2-4-9)7-13-6-5-12-10(13)14/h1-6H,7H2,(H,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025799

(1-(3,5-Dichloro-4-methoxy-benzyl)-1,3-dihydro-imid...)Show InChI InChI=1S/C11H10Cl2N2OS/c1-16-10-8(12)4-7(5-9(10)13)6-15-3-2-14-11(15)17/h2-5H,6H2,1H3,(H,14,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025804
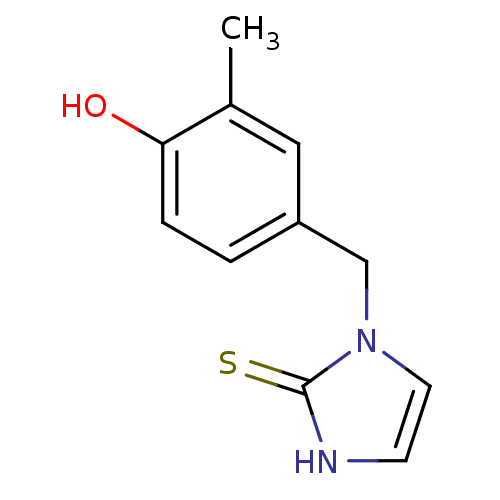
(1-(4-Hydroxy-3-methyl-benzyl)-1,3-dihydro-imidazol...)Show InChI InChI=1S/C11H12N2OS/c1-8-6-9(2-3-10(8)14)7-13-5-4-12-11(13)15/h2-6,14H,7H2,1H3,(H,12,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025787
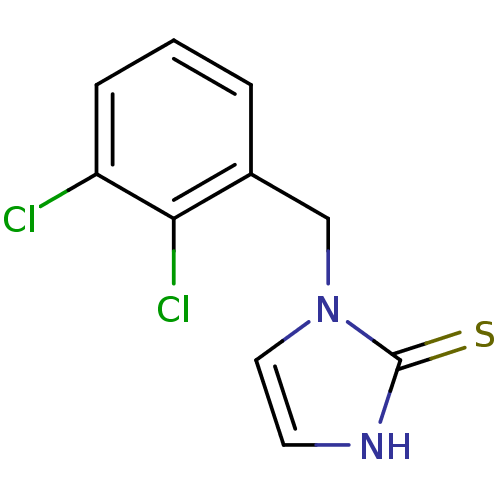
(1-(2,3-Dichloro-benzyl)-1,3-dihydro-imidazole-2-th...)Show InChI InChI=1S/C10H8Cl2N2S/c11-8-3-1-2-7(9(8)12)6-14-5-4-13-10(14)15/h1-5H,6H2,(H,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025807
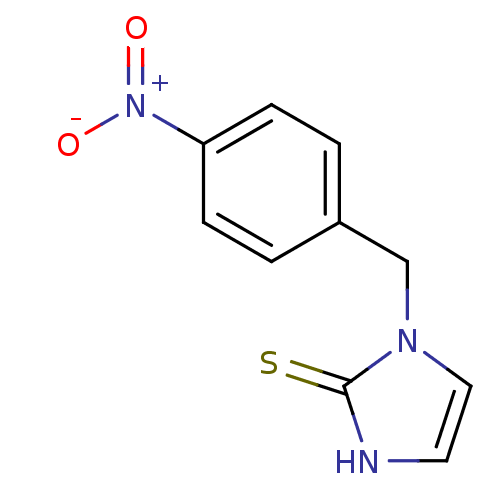
(1-(4-Nitro-benzyl)-1,3-dihydro-imidazole-2-thione ...)Show InChI InChI=1S/C10H9N3O2S/c14-13(15)9-3-1-8(2-4-9)7-12-6-5-11-10(12)16/h1-6H,7H2,(H,11,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50025808
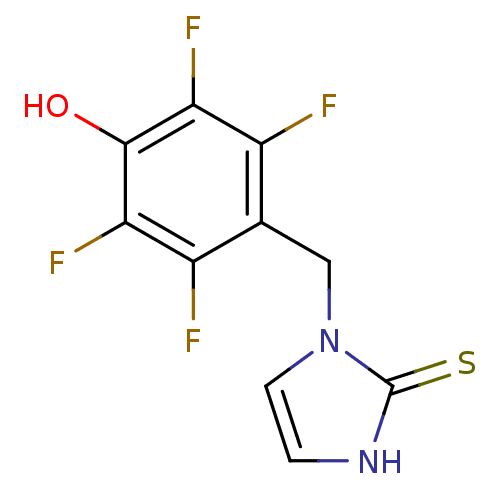
(1-(2,3,5,6-Tetrafluoro-4-hydroxy-benzyl)-1,3-dihyd...)Show InChI InChI=1S/C10H6F4N2OS/c11-5-4(3-16-2-1-15-10(16)18)6(12)8(14)9(17)7(5)13/h1-2,17H,3H2,(H,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50055205

(CHEMBL136669 | Trimethyl-(2-methyl-5-propionyloxy-...)Show InChI InChI=1S/C13H20NO2/c1-6-13(15)16-11-8-7-10(2)12(9-11)14(3,4)5/h7-9H,6H2,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition acetylcholinesterase (AChE) enzyme. |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50406071

(CHEMBL63619)Show InChI InChI=1S/C12H14N2OS/c1-9-7-10(3-4-11(9)15-2)8-14-6-5-13-12(14)16/h3-7H,8H2,1-2H3,(H,13,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Homo sapiens (Human)) | BDBM50406074
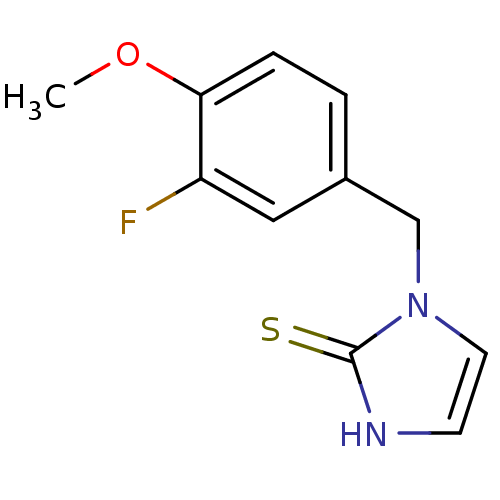
(CHEMBL302341)Show InChI InChI=1S/C11H11FN2OS/c1-15-10-3-2-8(6-9(10)12)7-14-5-4-13-11(14)16/h2-6H,7H2,1H3,(H,13,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Inhibition of dopamine beta-hydroxylase (DbetaH) enzyme |
J Med Chem 40: 4360-71 (1998)
Article DOI: 10.1021/jm970488n
BindingDB Entry DOI: 10.7270/Q2BZ6784 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data