 Found 52 hits Enz. Inhib. hit(s) with all data for entry = 50041455
Found 52 hits Enz. Inhib. hit(s) with all data for entry = 50041455 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411015
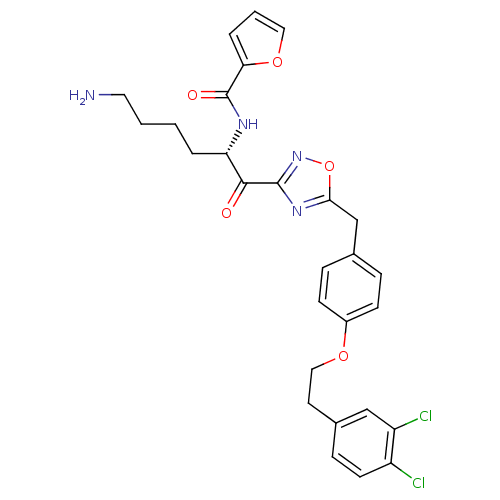
(CHEMBL539086)Show SMILES NCCCC[C@H](NC(=O)c1ccco1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H28Cl2N4O5/c29-21-11-8-19(16-22(21)30)12-15-37-20-9-6-18(7-10-20)17-25-33-27(34-39-25)26(35)23(4-1-2-13-31)32-28(36)24-5-3-14-38-24/h3,5-11,14,16,23H,1-2,4,12-13,15,17,31H2,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411006
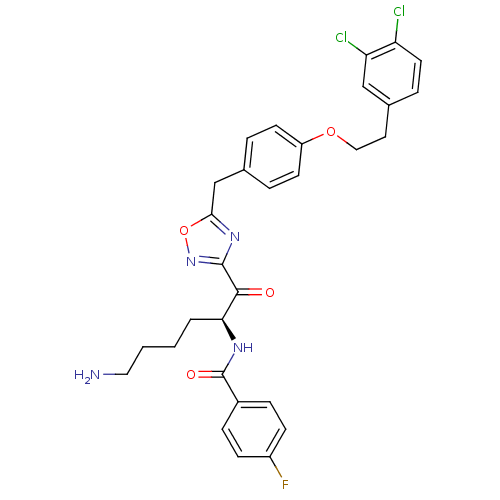
(CHEMBL539088)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-13-6-20(17-25(24)32)14-16-40-23-11-4-19(5-12-23)18-27-36-29(37-41-27)28(38)26(3-1-2-15-34)35-30(39)21-7-9-22(33)10-8-21/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411011
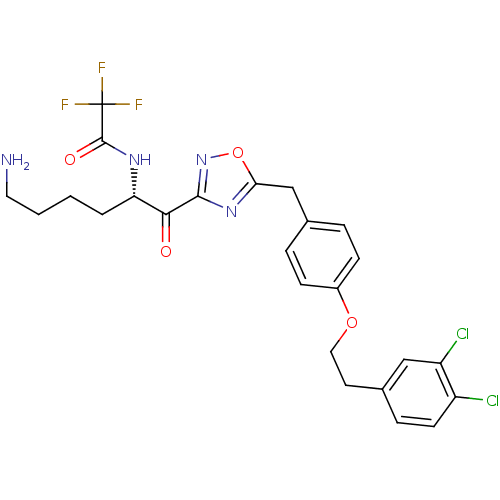
(CHEMBL537868)Show SMILES NCCCC[C@H](NC(=O)C(F)(F)F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C25H25Cl2F3N4O4/c26-18-9-6-16(13-19(18)27)10-12-37-17-7-4-15(5-8-17)14-21-33-23(34-38-21)22(35)20(3-1-2-11-31)32-24(36)25(28,29)30/h4-9,13,20H,1-3,10-12,14,31H2,(H,32,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411017
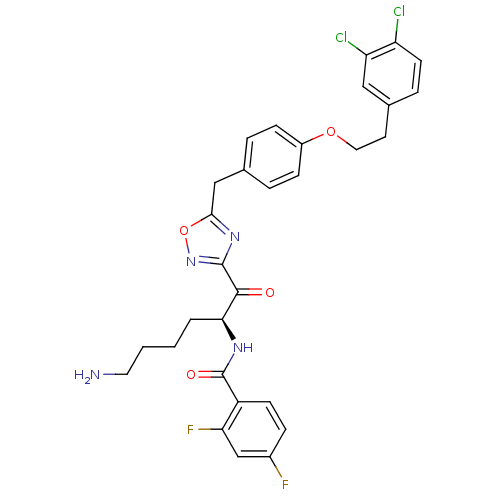
(CHEMBL539603)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-23-11-6-19(15-24(23)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)22-10-7-20(33)17-25(22)34/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411009
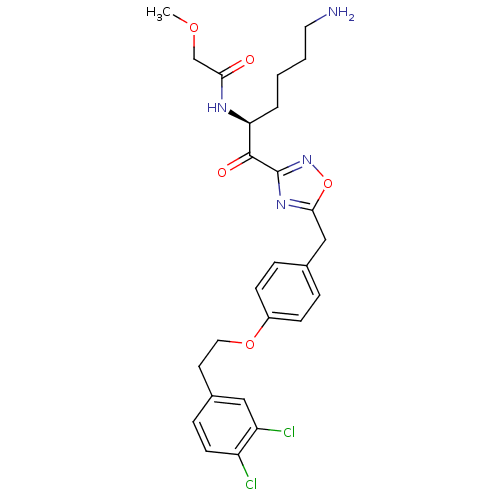
(CHEMBL538574)Show SMILES COCC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H30Cl2N4O5/c1-35-16-23(33)30-22(4-2-3-12-29)25(34)26-31-24(37-32-26)15-17-5-8-19(9-6-17)36-13-11-18-7-10-20(27)21(28)14-18/h5-10,14,22H,2-4,11-13,15-16,29H2,1H3,(H,30,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411002
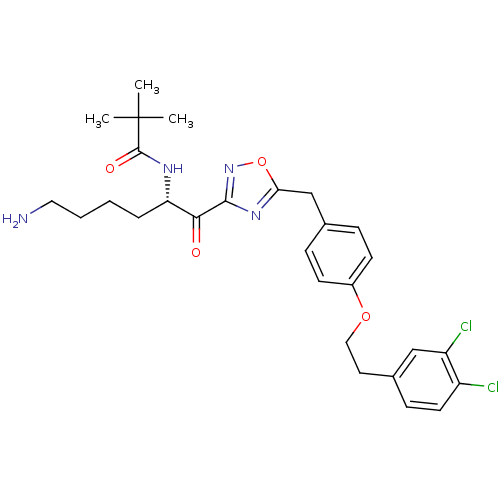
(CHEMBL556994)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H34Cl2N4O4/c1-28(2,3)27(36)32-23(6-4-5-14-31)25(35)26-33-24(38-34-26)17-18-7-10-20(11-8-18)37-15-13-19-9-12-21(29)22(30)16-19/h7-12,16,23H,4-6,13-15,17,31H2,1-3H3,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411013
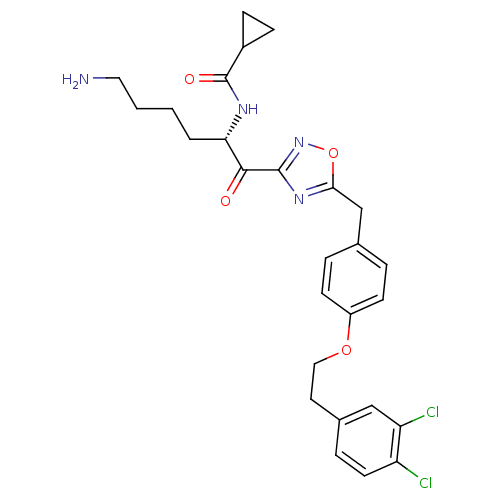
(CHEMBL534493)Show SMILES NCCCC[C@H](NC(=O)C1CC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H30Cl2N4O4/c28-21-11-6-18(15-22(21)29)12-14-36-20-9-4-17(5-10-20)16-24-32-26(33-37-24)25(34)23(3-1-2-13-30)31-27(35)19-7-8-19/h4-6,9-11,15,19,23H,1-3,7-8,12-14,16,30H2,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410996
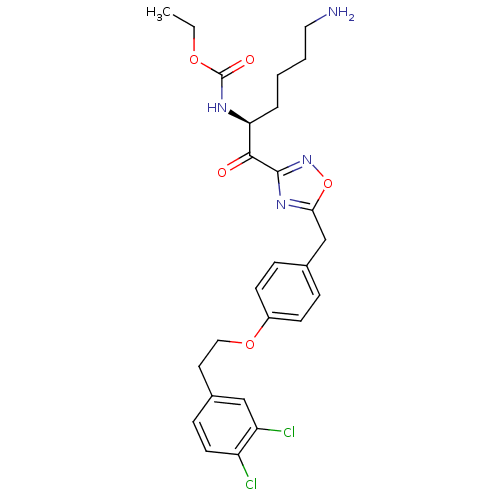
(CHEMBL537647)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H30Cl2N4O5/c1-2-35-26(34)30-22(5-3-4-13-29)24(33)25-31-23(37-32-25)16-17-6-9-19(10-7-17)36-14-12-18-8-11-20(27)21(28)15-18/h6-11,15,22H,2-5,12-14,16,29H2,1H3,(H,30,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411005
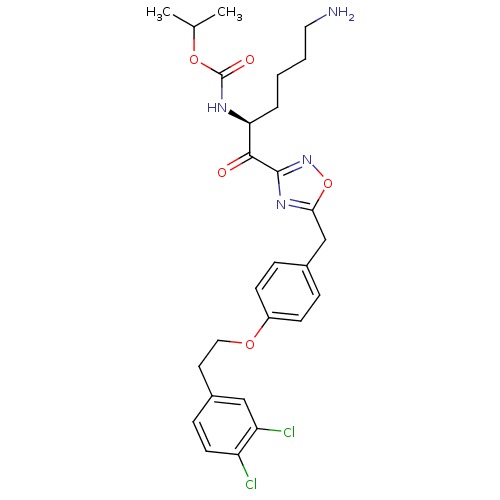
(CHEMBL537646)Show SMILES CC(C)OC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O5/c1-17(2)37-27(35)31-23(5-3-4-13-30)25(34)26-32-24(38-33-26)16-18-6-9-20(10-7-18)36-14-12-19-8-11-21(28)22(29)15-19/h6-11,15,17,23H,3-5,12-14,16,30H2,1-2H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411019
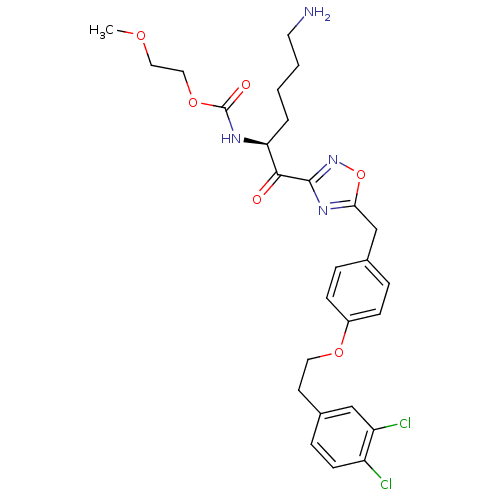
(CHEMBL558199)Show SMILES COCCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O6/c1-36-14-15-38-27(35)31-23(4-2-3-12-30)25(34)26-32-24(39-33-26)17-18-5-8-20(9-6-18)37-13-11-19-7-10-21(28)22(29)16-19/h5-10,16,23H,2-4,11-15,17,30H2,1H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411001
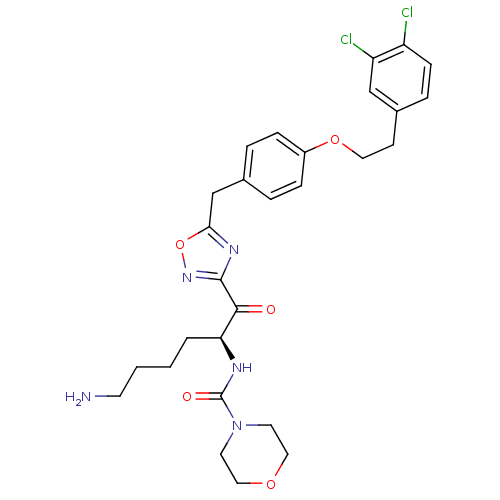
(CHEMBL558763)Show SMILES NCCCC[C@H](NC(=O)N1CCOCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H33Cl2N5O5/c29-22-9-6-20(17-23(22)30)10-14-39-21-7-4-19(5-8-21)18-25-33-27(34-40-25)26(36)24(3-1-2-11-31)32-28(37)35-12-15-38-16-13-35/h4-9,17,24H,1-3,10-16,18,31H2,(H,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411008
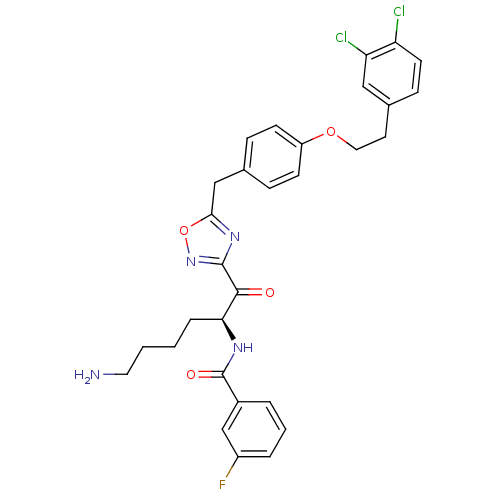
(CHEMBL535836)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-12-9-20(16-25(24)32)13-15-40-23-10-7-19(8-11-23)17-27-36-29(37-41-27)28(38)26(6-1-2-14-34)35-30(39)21-4-3-5-22(33)18-21/h3-5,7-12,16,18,26H,1-2,6,13-15,17,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411016
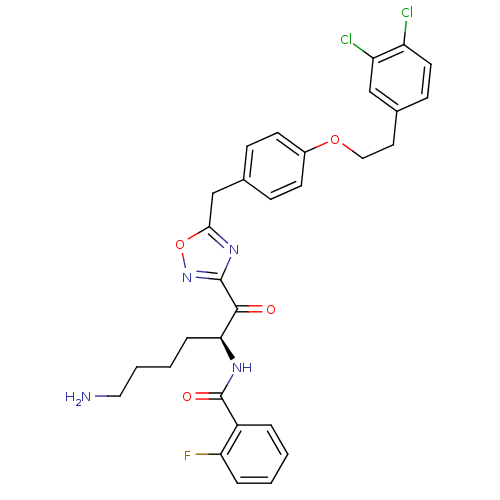
(CHEMBL559161)Show SMILES NCCCC[C@H](NC(=O)c1ccccc1F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-23-13-10-20(17-24(23)32)14-16-40-21-11-8-19(9-12-21)18-27-36-29(37-41-27)28(38)26(7-3-4-15-34)35-30(39)22-5-1-2-6-25(22)33/h1-2,5-6,8-13,17,26H,3-4,7,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411007
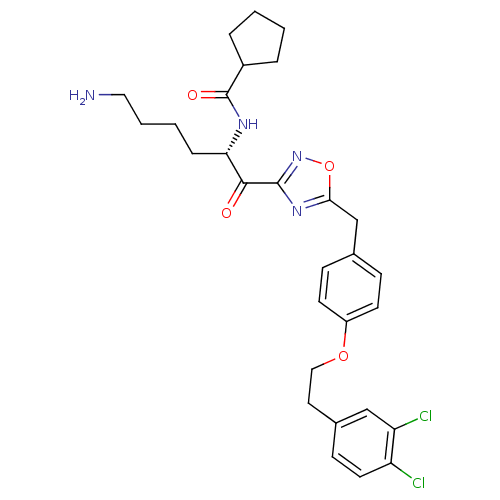
(CHEMBL534492)Show SMILES NCCCC[C@H](NC(=O)C1CCCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H34Cl2N4O4/c30-23-13-10-20(17-24(23)31)14-16-38-22-11-8-19(9-12-22)18-26-34-28(35-39-26)27(36)25(7-3-4-15-32)33-29(37)21-5-1-2-6-21/h8-13,17,21,25H,1-7,14-16,18,32H2,(H,33,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411018
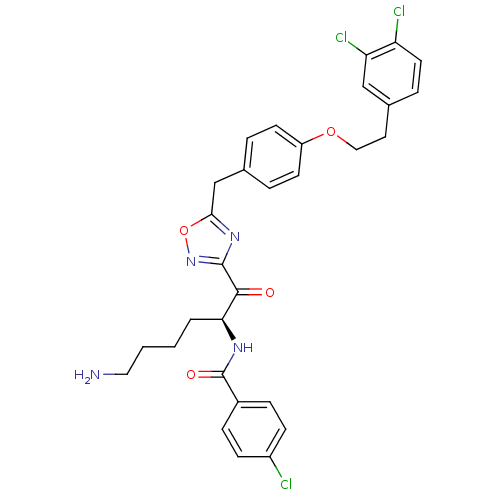
(CHEMBL559184)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl3N4O4/c31-22-9-7-21(8-10-22)30(39)35-26(3-1-2-15-34)28(38)29-36-27(41-37-29)18-19-4-11-23(12-5-19)40-16-14-20-6-13-24(32)25(33)17-20/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411010

(CHEMBL535163)Show SMILES CC(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O4/c1-17(2)27(35)31-23(5-3-4-13-30)25(34)26-32-24(37-33-26)16-18-6-9-20(10-7-18)36-14-12-19-8-11-21(28)22(29)15-19/h6-11,15,17,23H,3-5,12-14,16,30H2,1-2H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410995
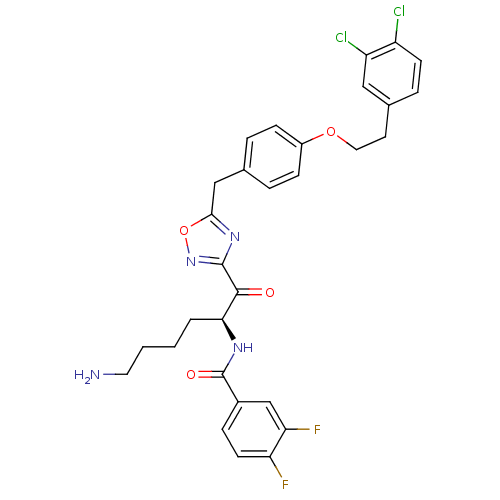
(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410998
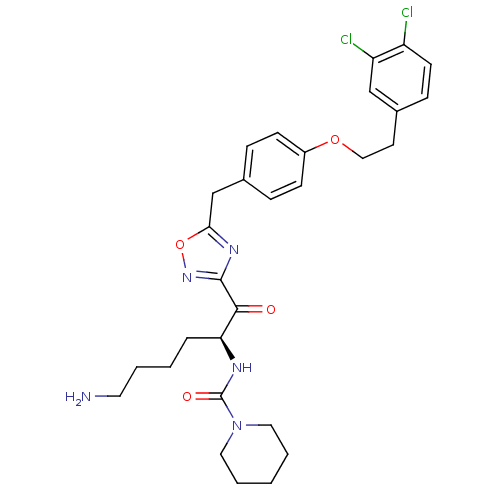
(CHEMBL535617)Show SMILES NCCCC[C@H](NC(=O)N1CCCCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H35Cl2N5O4/c30-23-12-9-21(18-24(23)31)13-17-39-22-10-7-20(8-11-22)19-26-34-28(35-40-26)27(37)25(6-2-3-14-32)33-29(38)36-15-4-1-5-16-36/h7-12,18,25H,1-6,13-17,19,32H2,(H,33,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411004
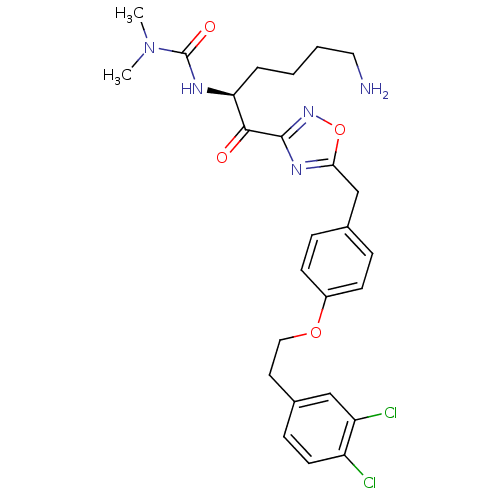
(CHEMBL535386)Show SMILES CN(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H31Cl2N5O4/c1-33(2)26(35)30-22(5-3-4-13-29)24(34)25-31-23(37-32-25)16-17-6-9-19(10-7-17)36-14-12-18-8-11-20(27)21(28)15-18/h6-11,15,22H,3-5,12-14,16,29H2,1-2H3,(H,30,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411000
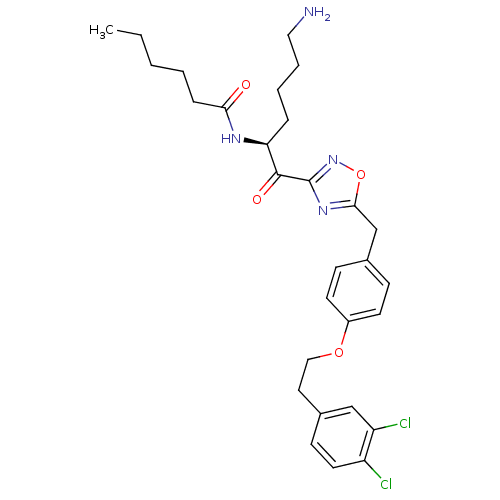
(CHEMBL558178)Show SMILES CCCCCC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H36Cl2N4O4/c1-2-3-4-8-26(36)33-25(7-5-6-16-32)28(37)29-34-27(39-35-29)19-20-9-12-22(13-10-20)38-17-15-21-11-14-23(30)24(31)18-21/h9-14,18,25H,2-8,15-17,19,32H2,1H3,(H,33,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410999

(CHEMBL557979)Show SMILES CC(C)(C)COC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H36Cl2N4O5/c1-29(2,3)18-39-28(37)33-24(6-4-5-14-32)26(36)27-34-25(40-35-27)17-19-7-10-21(11-8-19)38-15-13-20-9-12-22(30)23(31)16-20/h7-12,16,24H,4-6,13-15,17-18,32H2,1-3H3,(H,33,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410997
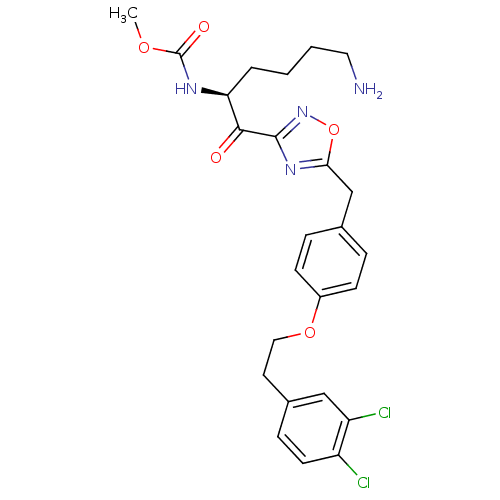
(CHEMBL557987)Show SMILES COC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C25H28Cl2N4O5/c1-34-25(33)29-21(4-2-3-12-28)23(32)24-30-22(36-31-24)15-16-5-8-18(9-6-16)35-13-11-17-7-10-19(26)20(27)14-17/h5-10,14,21H,2-4,11-13,15,28H2,1H3,(H,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410994
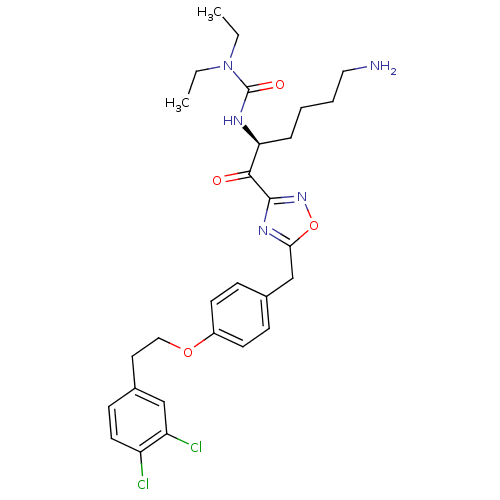
(CHEMBL536290)Show SMILES CCN(CC)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H35Cl2N5O4/c1-3-35(4-2)28(37)32-24(7-5-6-15-31)26(36)27-33-25(39-34-27)18-19-8-11-21(12-9-19)38-16-14-20-10-13-22(29)23(30)17-20/h8-13,17,24H,3-7,14-16,18,31H2,1-2H3,(H,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411003
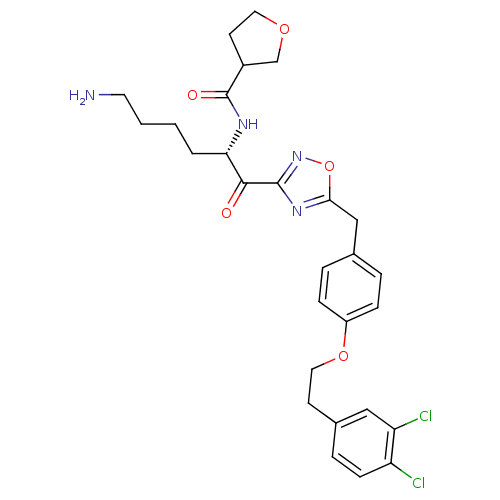
(CHEMBL536973)Show SMILES NCCCC[C@H](NC(=O)C1CCOC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H32Cl2N4O5/c29-22-9-6-19(15-23(22)30)10-14-38-21-7-4-18(5-8-21)16-25-33-27(34-39-25)26(35)24(3-1-2-12-31)32-28(36)20-11-13-37-17-20/h4-9,15,20,24H,1-3,10-14,16-17,31H2,(H,32,36)/t20?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411012
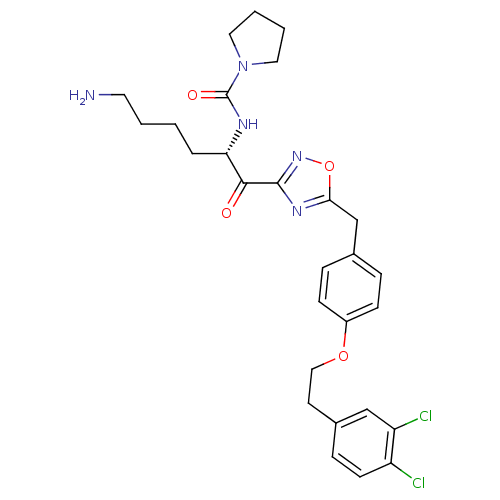
(CHEMBL535385)Show SMILES NCCCC[C@H](NC(=O)N1CCCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H33Cl2N5O4/c29-22-11-8-20(17-23(22)30)12-16-38-21-9-6-19(7-10-21)18-25-33-27(34-39-25)26(36)24(5-1-2-13-31)32-28(37)35-14-3-4-15-35/h6-11,17,24H,1-5,12-16,18,31H2,(H,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411014
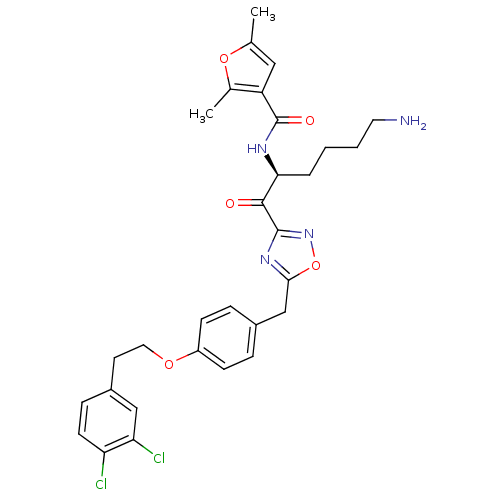
(CHEMBL538085)Show SMILES Cc1cc(C(=O)N[C@@H](CCCCN)C(=O)c2noc(Cc3ccc(OCCc4ccc(Cl)c(Cl)c4)cc3)n2)c(C)o1 |r| Show InChI InChI=1S/C30H32Cl2N4O5/c1-18-15-23(19(2)40-18)30(38)34-26(5-3-4-13-33)28(37)29-35-27(41-36-29)17-20-6-9-22(10-7-20)39-14-12-21-8-11-24(31)25(32)16-21/h6-11,15-16,26H,3-5,12-14,17,33H2,1-2H3,(H,34,38)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase
(Mus musculus) | BDBM50411006

(CHEMBL539088)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-13-6-20(17-25(24)32)14-16-40-23-11-4-19(5-12-23)18-27-36-29(37-41-27)28(38)26(3-1-2-15-34)35-30(39)21-7-9-22(33)10-8-21/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of mouse tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase
(Mus musculus) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of mouse tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411006

(CHEMBL539088)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-13-6-20(17-25(24)32)14-16-40-23-11-4-19(5-12-23)18-27-36-29(37-41-27)28(38)26(3-1-2-15-34)35-30(39)21-7-9-22(33)10-8-21/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of monkey tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of monkey tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of kallikrein |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of kallikrein |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Granzyme K
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of granzyme K |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of factor 11a |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of factor 9a |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of chymase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of APC |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of factor 7a |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Granzyme K
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of granzyme K |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of APC |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50410995

(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data