

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50411865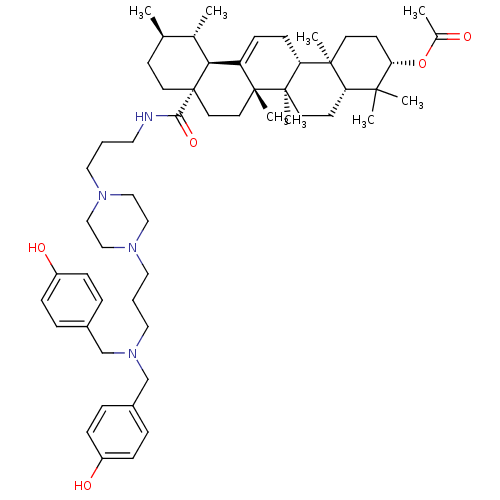 (CHEMBL261832) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS) Curated by ChEMBL | Assay Description Inhibition of beta-hematin formation | Bioorg Med Chem 16: 771-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.031 BindingDB Entry DOI: 10.7270/Q23N24MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM22985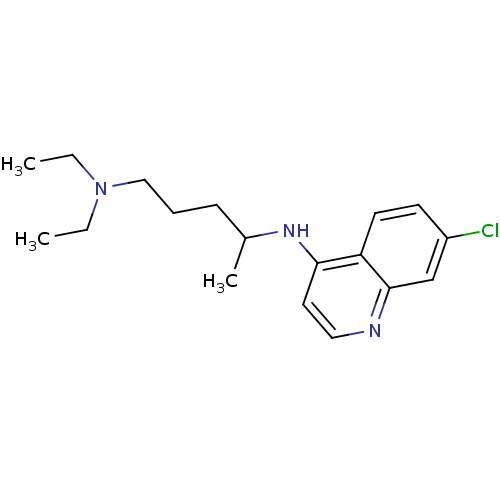 (Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS) Curated by ChEMBL | Assay Description Inhibition of beta-hematin formation | Bioorg Med Chem 16: 771-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.031 BindingDB Entry DOI: 10.7270/Q23N24MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50225900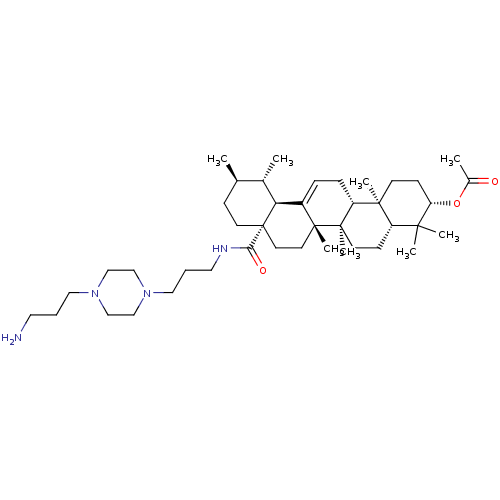 (CHEMBL270215 | N-{3-[4-(3-aminopropyl)piperazinyl]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS) Curated by ChEMBL | Assay Description Inhibition of beta-hematin formation | Bioorg Med Chem 16: 771-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.031 BindingDB Entry DOI: 10.7270/Q23N24MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS) Curated by ChEMBL | Assay Description Inhibition of beta-hematin formation | Bioorg Med Chem 16: 771-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.031 BindingDB Entry DOI: 10.7270/Q23N24MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine-rich protein PFHRP-II (Plasmodium falciparum) | BDBM50411864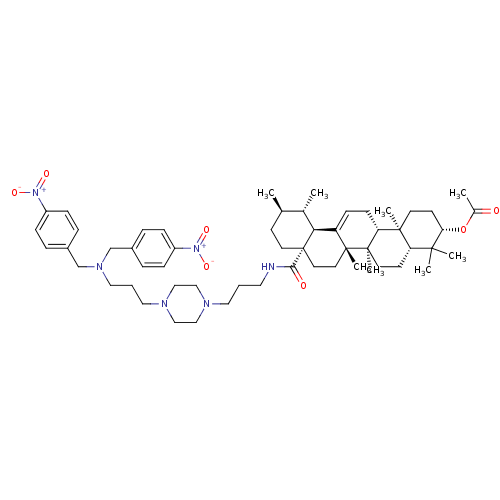 (CHEMBL436662) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS) Curated by ChEMBL | Assay Description Inhibition of beta-hematin formation | Bioorg Med Chem 16: 771-82 (2008) Article DOI: 10.1016/j.bmc.2007.10.031 BindingDB Entry DOI: 10.7270/Q23N24MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||