

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50360383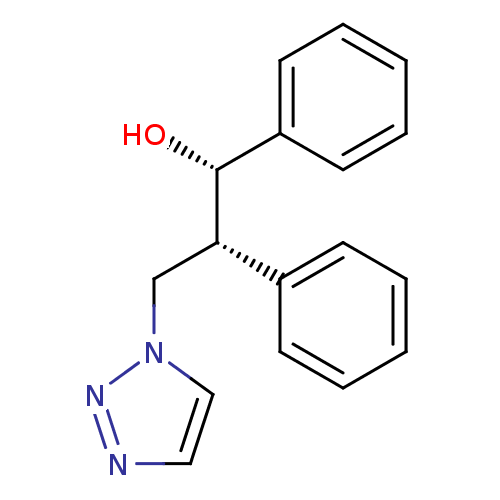 (CHEMBL1933700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360381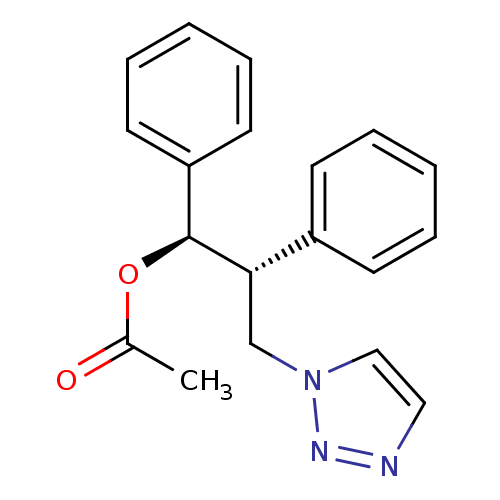 (CHEMBL1933694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360382 (CHEMBL1933699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360380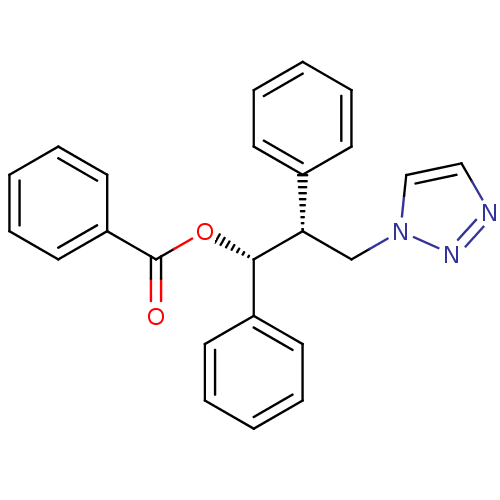 (CHEMBL1933693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360384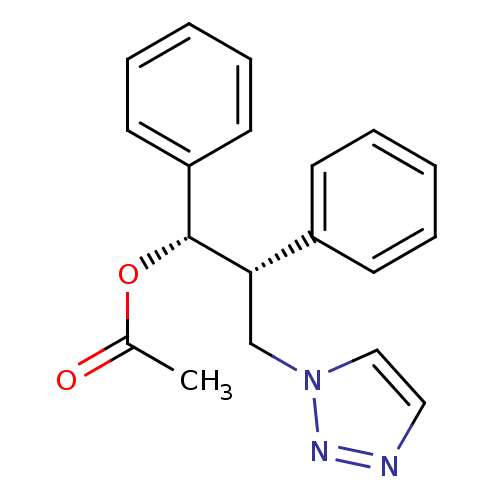 (CHEMBL1933701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360378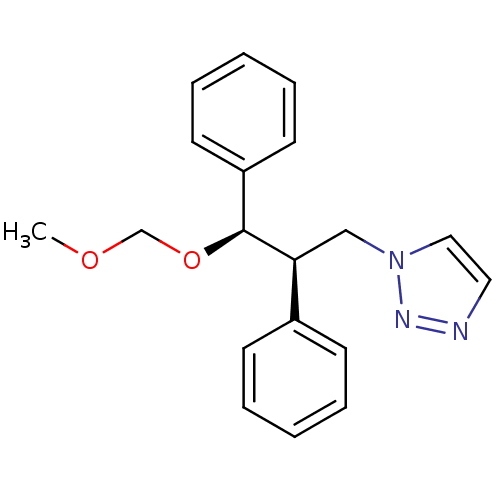 (CHEMBL1933690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50360379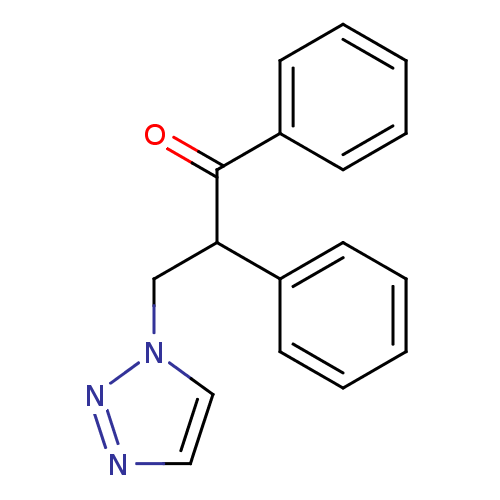 (CHEMBL1933692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome... | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50360379 (CHEMBL1933692) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50360380 (CHEMBL1933693) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50360383 (CHEMBL1933700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50360378 (CHEMBL1933690) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 22: 718-22 (2011) Article DOI: 10.1016/j.bmcl.2011.10.039 BindingDB Entry DOI: 10.7270/Q2PV6KS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||