 Found 133 hits Enz. Inhib. hit(s) with all data for entry = 50037261
Found 133 hits Enz. Inhib. hit(s) with all data for entry = 50037261 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50037187
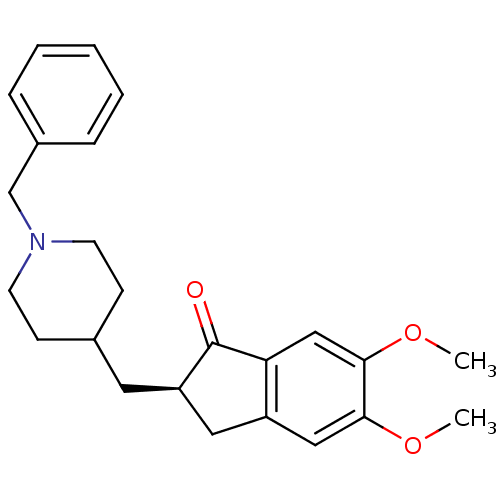
((2R)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimeth...)Show SMILES COc1cc2C[C@@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Binding affinity tested against acetylcholinesterase in Torpedo californica |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50037176
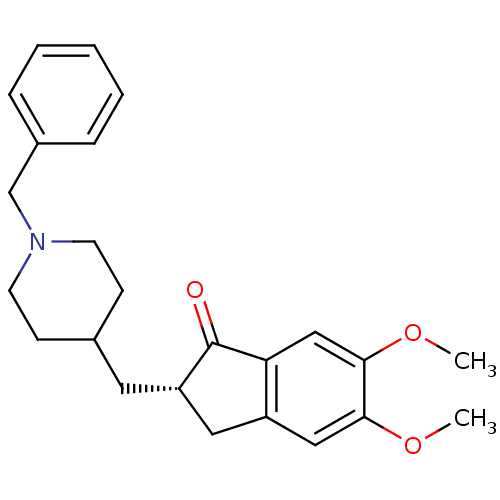
((2S)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimeth...)Show SMILES COc1cc2C[C@H](CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Binding affinity tested against acetylcholinesterase in Torpedo californica |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50409820

(CHEMBL2114386)Show SMILES Cc1cccc2CO[P@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |r,c:15| Show InChI InChI=1S/C18H19N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-8,14-15H,9-10H2,1-2H3,(H,19,21,22)/t14-,15+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146850
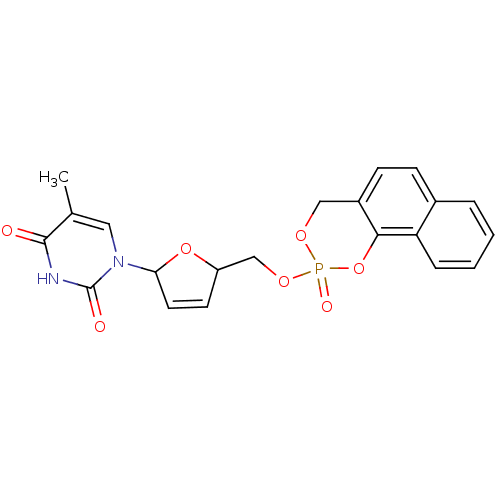
(5-Methyl-1-[5-(3-oxo-1H-2,4-dioxa-3lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4ccc5ccccc5c4O3)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)18-9-8-16(29-18)12-28-31(26)27-11-15-7-6-14-4-2-3-5-17(14)19(15)30-31/h2-10,16,18H,11-12H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146849
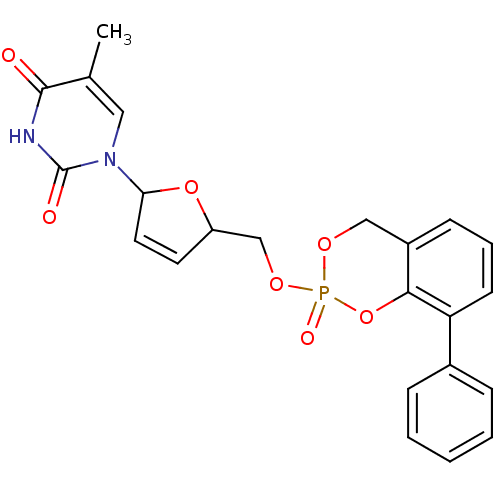
(5-Methyl-1-[5-(2-oxo-8-phenyl-4H-2lambda*5*-benzo[...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cccc(-c5ccccc5)c4O3)C=C2)c(=O)[nH]c1=O |c:29| Show InChI InChI=1S/C23H21N2O7P/c1-15-12-25(23(27)24-22(15)26)20-11-10-18(31-20)14-30-33(28)29-13-17-8-5-9-19(21(17)32-33)16-6-3-2-4-7-16/h2-12,18,20H,13-14H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146859

(5-Methyl-1-[5-(2-oxo-4H-1,3-dioxa-2lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc5ccccc5cc4O3)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)19-7-6-17(29-19)12-28-31(26)27-11-16-8-14-4-2-3-5-15(14)9-18(16)30-31/h2-10,17,19H,11-12H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146881

(1-[5-(5-Chloro-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4c(Cl)cccc4O3)C=C2)c(=O)[nH]c1=O |c:23| Show InChI InChI=1S/C17H16ClN2O7P/c1-10-7-20(17(22)19-16(10)21)15-6-5-11(26-15)8-24-28(23)25-9-12-13(18)3-2-4-14(12)27-28/h2-7,11,15H,8-9H2,1H3,(H,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50064050
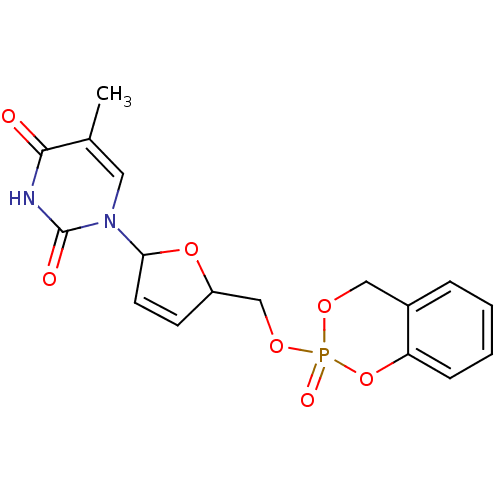
(5-Methyl-1-[(2R,5S)-5-(2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4ccccc4O3)C=C2)c(=O)[nH]c1=O |c:22| Show InChI InChI=1S/C17H17N2O7P/c1-11-8-19(17(21)18-16(11)20)15-7-6-13(25-15)10-24-27(22)23-9-12-4-2-3-5-14(12)26-27/h2-8,13,15H,9-10H2,1H3,(H,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50076440
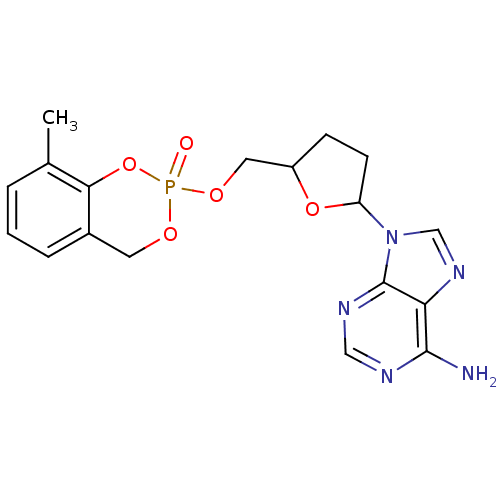
(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3CCC(O3)n3cnc4c(N)ncnc34)Oc12 Show InChI InChI=1S/C18H20N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-4,9-10,13-14H,5-8H2,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146872
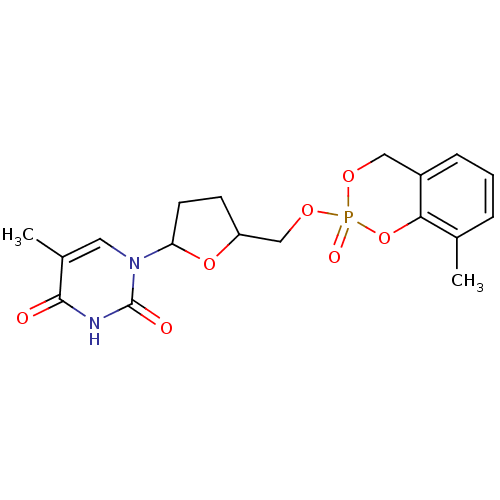
(5-Methyl-1-[5-(8-methyl-2-oxo-4H-2lambda*5*-benzo[...)Show SMILES Cc1cccc2COP(=O)(OCC3CCC(O3)n3cc(C)c(=O)[nH]c3=O)Oc12 Show InChI InChI=1S/C18H21N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-5,8,14-15H,6-7,9-10H2,1-2H3,(H,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422510
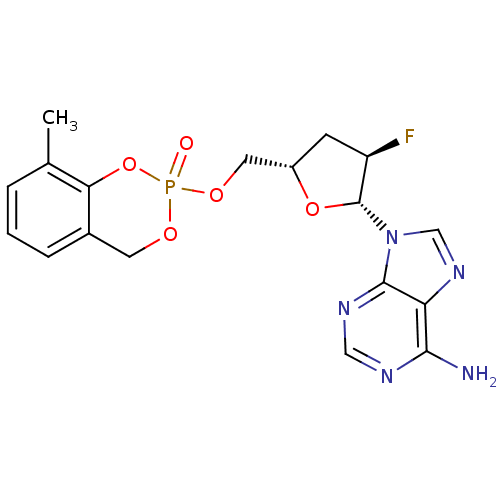
(CHEMBL2112391)Show SMILES Cc1cccc2COP(=O)(OC[C@@H]3C[C@@H](F)[C@@H](O3)n3cnc4c(N)ncnc34)Oc12 |r| Show InChI InChI=1S/C18H19FN5O5P/c1-10-3-2-4-11-6-26-30(25,29-15(10)11)27-7-12-5-13(19)18(28-12)24-9-23-14-16(20)21-8-22-17(14)24/h2-4,8-9,12-13,18H,5-7H2,1H3,(H2,20,21,22)/t12-,13+,18+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50409820

(CHEMBL2114386)Show SMILES Cc1cccc2CO[P@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |r,c:15| Show InChI InChI=1S/C18H19N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-8,14-15H,9-10H2,1-2H3,(H,19,21,22)/t14-,15+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146867

(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3OC(C=C3)n3cnc4c(N)ncnc34)Oc12 |c:15| Show InChI InChI=1S/C18H18N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-6,9-10,13-14H,7-8H2,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146868

((R)-N*6*-Cyclopropyl-9-[(R)-4-(8-methyl-2-oxo-4H-2...)Show SMILES Cc1cccc2COP(=O)(OC[C@@H]3CC(C=C3)n3cnc4c(NC5CC5)nc(N)nc34)Oc12 |c:15| Show InChI InChI=1S/C22H25N6O4P/c1-13-3-2-4-15-11-31-33(29,32-19(13)15)30-10-14-5-8-17(9-14)28-12-24-18-20(25-16-6-7-16)26-22(23)27-21(18)28/h2-5,8,12,14,16-17H,6-7,9-11H2,1H3,(H3,23,25,26,27)/t14-,17?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146870

(1-[5-(5-Chloro-4-methyl-2-oxo-4H-2lambda*5*-benzo[...)Show SMILES CC1OP(=O)(OCC2OC(C=C2)n2cc(C)c(=O)[nH]c2=O)Oc2cccc(Cl)c12 |c:10| Show InChI InChI=1S/C18H18ClN2O7P/c1-10-8-21(18(23)20-17(10)22)15-7-6-12(26-15)9-25-29(24)27-11(2)16-13(19)4-3-5-14(16)28-29/h3-8,11-12,15H,9H2,1-2H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422509
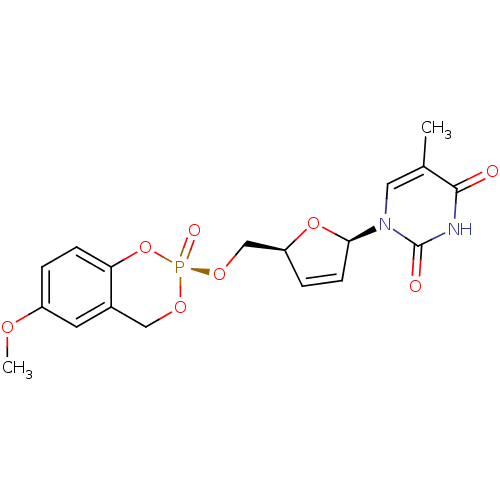
(CHEMBL2114383)Show SMILES COc1ccc2O[P@@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |r,c:14| Show InChI InChI=1S/C18H19N2O8P/c1-11-8-20(18(22)19-17(11)21)16-6-4-14(27-16)10-26-29(23)25-9-12-7-13(24-2)3-5-15(12)28-29/h3-8,14,16H,9-10H2,1-2H3,(H,19,21,22)/t14-,16+,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146865
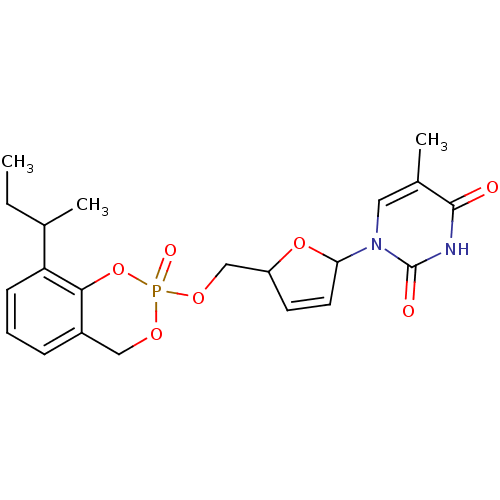
(1-[5-(8-sec-Butyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]...)Show SMILES CCC(C)c1cccc2COP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |c:18| Show InChI InChI=1S/C21H25N2O7P/c1-4-13(2)17-7-5-6-15-11-27-31(26,30-19(15)17)28-12-16-8-9-18(29-16)23-10-14(3)20(24)22-21(23)25/h5-10,13,16,18H,4,11-12H2,1-3H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50076440

(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3CCC(O3)n3cnc4c(N)ncnc34)Oc12 Show InChI InChI=1S/C18H20N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-4,9-10,13-14H,5-8H2,1H3,(H2,19,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards mouse butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146886
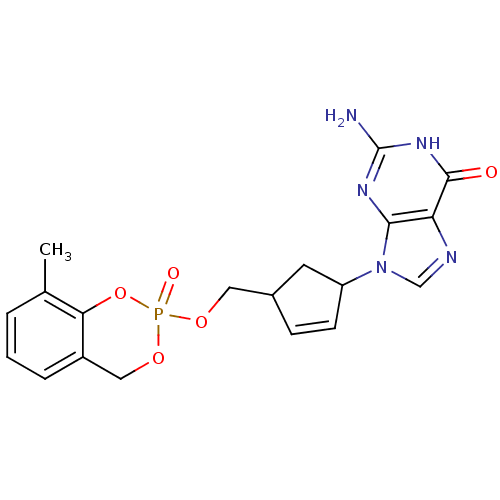
(2-Amino-9-[4-(8-methyl-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cccc2COP(=O)(OCC3CC(C=C3)n3cnc4c3nc(N)[nH]c4=O)Oc12 |c:15| Show InChI InChI=1S/C19H20N5O5P/c1-11-3-2-4-13-9-28-30(26,29-16(11)13)27-8-12-5-6-14(7-12)24-10-21-15-17(24)22-19(20)23-18(15)25/h2-6,10,12,14H,7-9H2,1H3,(H3,20,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422515

(CHEMBL2114301)Show SMILES Cc1cn([C@@H]2O[C@H](CO[P@]3(=O)OCc4cccc(c4O3)C(C)(C)C)C=C2)c(=O)[nH]c1=O |r,c:26| Show InChI InChI=1S/C21H25N2O7P/c1-13-10-23(20(25)22-19(13)24)17-9-8-15(29-17)12-28-31(26)27-11-14-6-5-7-16(18(14)30-31)21(2,3)4/h5-10,15,17H,11-12H2,1-4H3,(H,22,24,25)/t15-,17+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146873
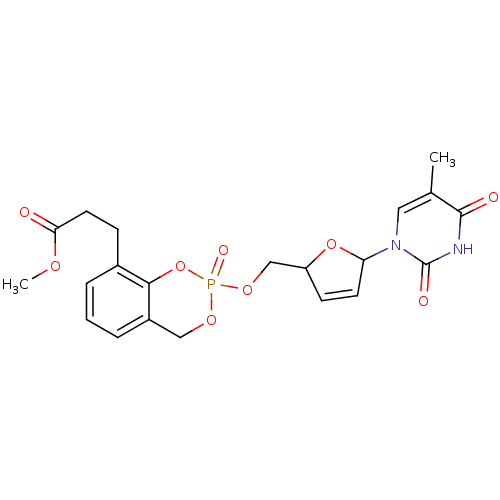
(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES COC(=O)CCc1cccc2COP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |c:20| Show InChI InChI=1S/C21H23N2O9P/c1-13-10-23(21(26)22-20(13)25)17-8-7-16(31-17)12-30-33(27)29-11-15-5-3-4-14(19(15)32-33)6-9-18(24)28-2/h3-5,7-8,10,16-17H,6,9,11-12H2,1-2H3,(H,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146864
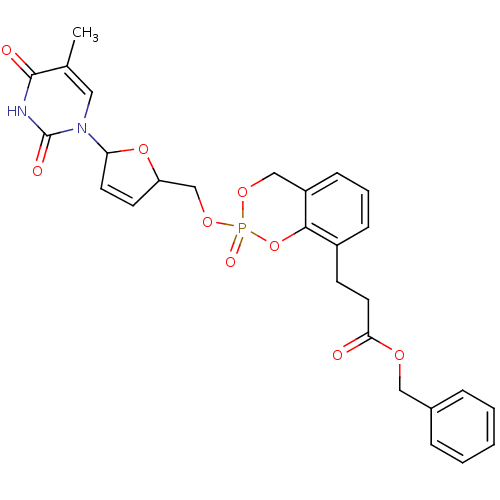
(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cccc(CCC(=O)OCc5ccccc5)c4O3)C=C2)c(=O)[nH]c1=O |c:35| Show InChI InChI=1S/C27H27N2O9P/c1-18-14-29(27(32)28-26(18)31)23-12-11-22(37-23)17-36-39(33)35-16-21-9-5-8-20(25(21)38-39)10-13-24(30)34-15-19-6-3-2-4-7-19/h2-9,11-12,14,22-23H,10,13,15-17H2,1H3,(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146877

(1-[5-(6-sec-Butyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]...)Show SMILES CCC(C)c1ccc2OP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |c:16| Show InChI InChI=1S/C21H25N2O7P/c1-4-13(2)15-5-7-18-16(9-15)11-27-31(26,30-18)28-12-17-6-8-19(29-17)23-10-14(3)20(24)22-21(23)25/h5-10,13,17,19H,4,11-12H2,1-3H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146876

(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cccc(CCC(=O)OC(C)(C)C)c4O3)C=C2)c(=O)[nH]c1=O |c:31| Show InChI InChI=1S/C24H29N2O9P/c1-15-12-26(23(29)25-22(15)28)19-10-9-18(33-19)14-32-36(30)31-13-17-7-5-6-16(21(17)35-36)8-11-20(27)34-24(2,3)4/h5-7,9-10,12,18-19H,8,11,13-14H2,1-4H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422516

(CHEMBL2114303)Show SMILES O[C@H]1C[C@@H](O[C@@H]1CO[P@]1(=O)OCc2cccc(-c3ccccc3)c2O1)N1CC(=CCBr)C(=O)NC1=O |r,w:28.32| Show InChI InChI=1S/C24H24BrN2O8P/c25-10-9-16-12-27(24(30)26-23(16)29)21-11-19(28)20(34-21)14-33-36(31)32-13-17-7-4-8-18(22(17)35-36)15-5-2-1-3-6-15/h1-9,19-21,28H,10-14H2,(H,26,29,30)/t19-,20+,21+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146889

(5-Methyl-1-[5-(2-oxo-4H-1,3-dioxa-2lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4c(O3)ccc3ccccc43)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)19-9-7-15(29-19)11-27-31(26)28-12-17-16-5-3-2-4-14(16)6-8-18(17)30-31/h2-10,15,19H,11-12H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146851
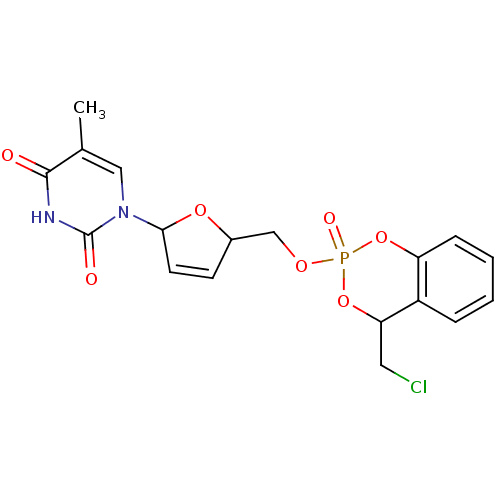
(1-[5-(4-Chloromethyl-2-oxo-4H-2lambda*5*-benzo[1,3...)Show SMILES Cc1cn(C2OC(COP3(=O)OC(CCl)c4ccccc4O3)C=C2)c(=O)[nH]c1=O |c:24| Show InChI InChI=1S/C18H18ClN2O7P/c1-11-9-21(18(23)20-17(11)22)16-7-6-12(26-16)10-25-29(24)27-14-5-3-2-4-13(14)15(8-19)28-29/h2-7,9,12,15-16H,8,10H2,1H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50422507

(CHEMBL375567)Show SMILES Cc1cn([C@@H]2O[C@H](COP3(=O)OCc4cccc(c4O3)C(C)(C)C)C=C2)c(=O)[nH]c1=O |c:26| Show InChI InChI=1S/C21H25N2O7P/c1-13-10-23(20(25)22-19(13)24)17-9-8-15(29-17)12-28-31(26)27-11-14-6-5-7-16(18(14)30-31)21(2,3)4/h5-10,15,17H,11-12H2,1-4H3,(H,22,24,25)/t15-,17+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146853

(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(CCC(=O)OC(C)(C)C)ccc4O3)C=C2)c(=O)[nH]c1=O |c:31| Show InChI InChI=1S/C24H29N2O9P/c1-15-12-26(23(29)25-22(15)28)20-9-7-18(33-20)14-32-36(30)31-13-17-11-16(5-8-19(17)35-36)6-10-21(27)34-24(2,3)4/h5,7-9,11-12,18,20H,6,10,13-14H2,1-4H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146856
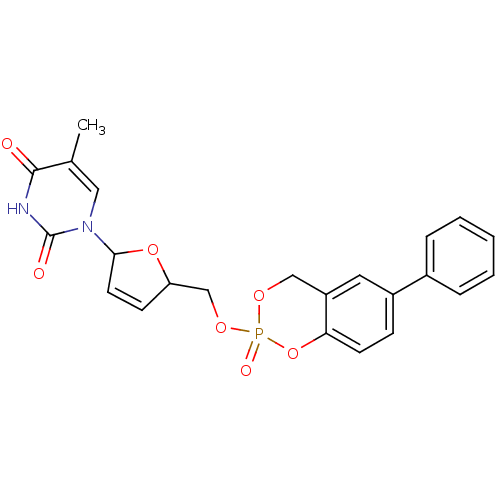
(5-Methyl-1-[5-(2-oxo-6-phenyl-4H-2lambda*5*-benzo[...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(ccc4O3)-c3ccccc3)C=C2)c(=O)[nH]c1=O |c:29| Show InChI InChI=1S/C23H21N2O7P/c1-15-12-25(23(27)24-22(15)26)21-10-8-19(31-21)14-30-33(28)29-13-18-11-17(7-9-20(18)32-33)16-5-3-2-4-6-16/h2-12,19,21H,13-14H2,1H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50064051

(5-Methyl-1-[(2R,5S)-5-(6-methyl-2-oxo-4H-2lambda*5...)Show SMILES Cc1ccc2OP(=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |r,c:13| Show InChI InChI=1S/C18H19N2O7P/c1-11-3-5-15-13(7-11)9-24-28(23,27-15)25-10-14-4-6-16(26-14)20-8-12(2)17(21)19-18(20)22/h3-8,14,16H,9-10H2,1-2H3,(H,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50409821

(CHEMBL2111583)Show SMILES Cc1cccc2COP(=O)(OC[C@@H]3C[C@H](F)[C@@H](O3)n3cnc4c(N)ncnc34)Oc12 |r| Show InChI InChI=1S/C18H19FN5O5P/c1-10-3-2-4-11-6-26-30(25,29-15(10)11)27-7-12-5-13(19)18(28-12)24-9-23-14-16(20)21-8-22-17(14)24/h2-4,8-9,12-13,18H,5-7H2,1H3,(H2,20,21,22)/t12-,13-,18+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146858
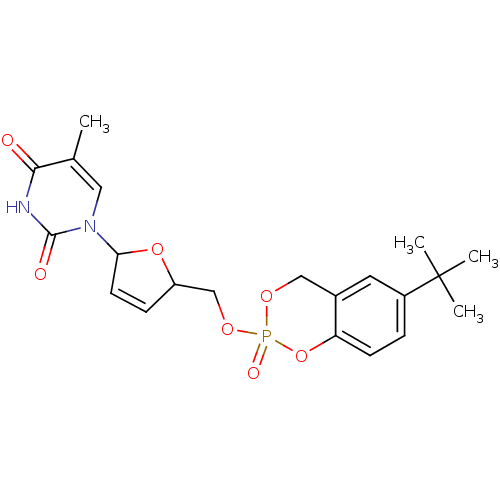
(1-[5-(6-tert-Butyl-2-oxo-4H-2lambda*5*-benzo[1,3,2...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(ccc4O3)C(C)(C)C)C=C2)c(=O)[nH]c1=O |c:26| Show InChI InChI=1S/C21H25N2O7P/c1-13-10-23(20(25)22-19(13)24)18-8-6-16(29-18)12-28-31(26)27-11-14-9-15(21(2,3)4)5-7-17(14)30-31/h5-10,16,18H,11-12H2,1-4H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146883
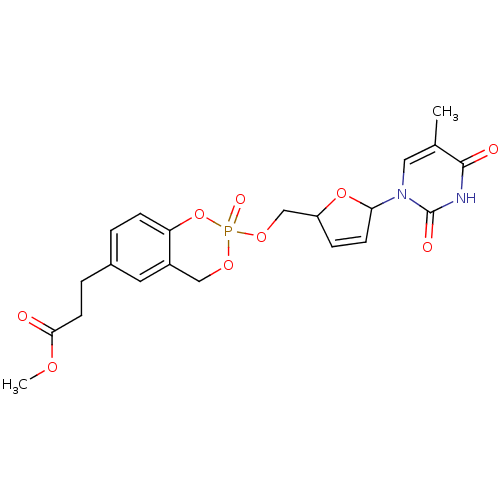
(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES COC(=O)CCc1ccc2OP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |c:18| Show InChI InChI=1S/C21H23N2O9P/c1-13-10-23(21(26)22-20(13)25)18-7-5-16(31-18)12-30-33(27)29-11-15-9-14(3-6-17(15)32-33)4-8-19(24)28-2/h3,5-7,9-10,16,18H,4,8,11-12H2,1-2H3,(H,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50146867

(9-[5-(8-Methyl-2-oxo-4H-2lambda*5*-benzo[1,3,2]dio...)Show SMILES Cc1cccc2COP(=O)(OCC3OC(C=C3)n3cnc4c(N)ncnc34)Oc12 |c:15| Show InChI InChI=1S/C18H18N5O5P/c1-11-3-2-4-12-7-25-29(24,28-16(11)12)26-8-13-5-6-14(27-13)23-10-22-15-17(19)20-9-21-18(15)23/h2-6,9-10,13-14H,7-8H2,1H3,(H2,19,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards mouse butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146861
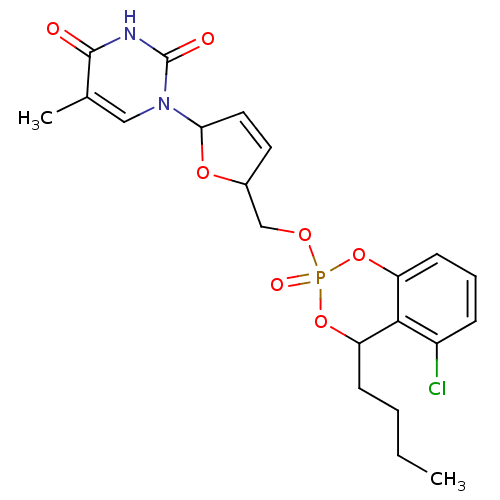
(1-[5-(4-Butyl-5-chloro-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES CCCCC1OP(=O)(OCC2OC(C=C2)n2cc(C)c(=O)[nH]c2=O)Oc2cccc(Cl)c12 |c:13| Show InChI InChI=1S/C21H24ClN2O7P/c1-3-4-7-16-19-15(22)6-5-8-17(19)31-32(27,30-16)28-12-14-9-10-18(29-14)24-11-13(2)20(25)23-21(24)26/h5-6,8-11,14,16,18H,3-4,7,12H2,1-2H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146866

(3-{2-[5-(5-Methyl-2,4-dioxo-3,4-dihydro-2H-pyrimid...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(CCC(=O)OCc5ccccc5)ccc4O3)C=C2)c(=O)[nH]c1=O |c:35| Show InChI InChI=1S/C27H27N2O9P/c1-18-14-29(27(32)28-26(18)31)24-11-9-22(37-24)17-36-39(33)35-16-21-13-19(7-10-23(21)38-39)8-12-25(30)34-15-20-5-3-2-4-6-20/h2-7,9-11,13-14,22,24H,8,12,15-17H2,1H3,(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146852
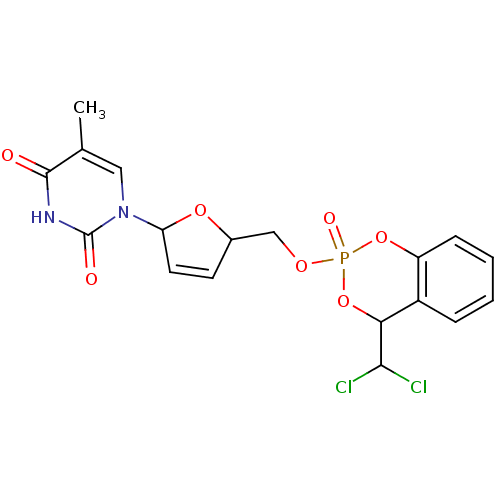
(1-[5-(4-Dichloromethyl-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cn(C2OC(COP3(=O)OC(C(Cl)Cl)c4ccccc4O3)C=C2)c(=O)[nH]c1=O |c:25| Show InChI InChI=1S/C18H17Cl2N2O7P/c1-10-8-22(18(24)21-17(10)23)14-7-6-11(27-14)9-26-30(25)28-13-5-3-2-4-12(13)15(29-30)16(19)20/h2-8,11,14-16H,9H2,1H3,(H,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146885

(5-Methyl-1-[5-(2-oxo-4-trichloromethyl-4H-2lambda*...)Show SMILES Cc1cn(C2OC(COP3(=O)OC(c4ccccc4O3)C(Cl)(Cl)Cl)C=C2)c(=O)[nH]c1=O |c:26| Show InChI InChI=1S/C18H16Cl3N2O7P/c1-10-8-23(17(25)22-16(10)24)14-7-6-11(28-14)9-27-31(26)29-13-5-3-2-4-12(13)15(30-31)18(19,20)21/h2-8,11,14-15H,9H2,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146878

(2-(6-Amino-purin-9-yl)-5-(8-methyl-2-oxo-4H-2lambd...)Show SMILES Cc1cccc2COP(=O)(OCC3CC(O)C(O3)n3cnc4c(N)ncnc34)Oc12 Show InChI InChI=1S/C18H20N5O6P/c1-10-3-2-4-11-6-26-30(25,29-15(10)11)27-7-12-5-13(24)18(28-12)23-9-22-14-16(19)20-8-21-17(14)23/h2-4,8-9,12-13,18,24H,5-7H2,1H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146880

(1-[4-Hydroxy-5-(8-methyl-2-oxo-4H-2lambda*5*-benzo...)Show SMILES Cc1cccc2COP(=O)(OC[C@H]3O[C@H](C[C@@H]3O)n3cc(C)c(=O)[nH]c3=O)Oc12 |r| Show InChI InChI=1S/C18H21N2O8P/c1-10-4-3-5-12-8-25-29(24,28-16(10)12)26-9-14-13(21)6-15(27-14)20-7-11(2)17(22)19-18(20)23/h3-5,7,13-15,21H,6,8-9H2,1-2H3,(H,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146862
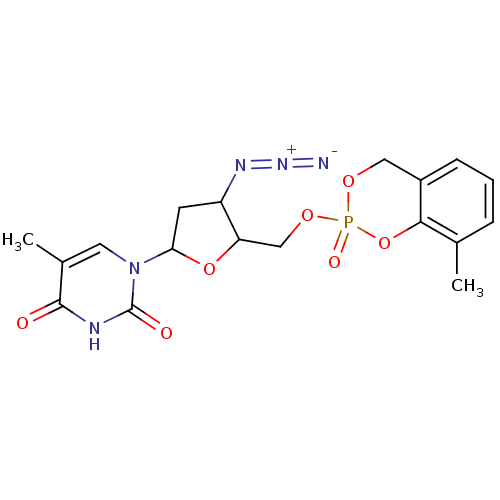
(1-[4-Azido-5-(8-methyl-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cccc2COP(=O)(OCC3OC(CC3N=[N+]=[N-])n3cc(C)c(=O)[nH]c3=O)Oc12 Show InChI InChI=1S/C18H20N5O7P/c1-10-4-3-5-12-8-27-31(26,30-16(10)12)28-9-14-13(21-22-19)6-15(29-14)23-7-11(2)17(24)20-18(23)25/h3-5,7,13-15H,6,8-9H2,1-2H3,(H,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50064055

(1-[5-(6,8-Dimethyl-2-oxo-4H-2lambda*5*-benzo[1,3,2...)Show SMILES Cc1cc(C)c2OP(=O)(OCC3OC(C=C3)n3cc(C)c(=O)[nH]c3=O)OCc2c1 |c:14| Show InChI InChI=1S/C19H21N2O7P/c1-11-6-12(2)17-14(7-11)9-25-29(24,28-17)26-10-15-4-5-16(27-15)21-8-13(3)18(22)20-19(21)23/h4-8,15-16H,9-10H2,1-3H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50146884

(1-[5-(6,8-Di-tert-butyl-5-fluoro-2-oxo-4H-2lambda*...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4c(F)c(cc(c4O3)C(C)(C)C)C(C)(C)C)C=C2)c(=O)[nH]c1=O |c:31| Show InChI InChI=1S/C25H32FN2O7P/c1-14-11-28(23(30)27-22(14)29)19-9-8-15(34-19)12-32-36(31)33-13-16-20(26)17(24(2,3)4)10-18(21(16)35-36)25(5,6)7/h8-11,15,19H,12-13H2,1-7H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50146858

(1-[5-(6-tert-Butyl-2-oxo-4H-2lambda*5*-benzo[1,3,2...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4cc(ccc4O3)C(C)(C)C)C=C2)c(=O)[nH]c1=O |c:26| Show InChI InChI=1S/C21H25N2O7P/c1-13-10-23(20(25)22-19(13)24)18-8-6-16(29-18)12-28-31(26)27-11-14-9-15(21(2,3)4)5-7-17(14)30-31/h5-10,16,18H,11-12H2,1-4H3,(H,22,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Electrophorus electricus acetylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50146889

(5-Methyl-1-[5-(2-oxo-4H-1,3-dioxa-2lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4c(O3)ccc3ccccc43)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)19-9-7-15(29-19)11-27-31(26)28-12-17-16-5-3-2-4-14(16)6-8-18(17)30-31/h2-10,15,19H,11-12H2,1H3,(H,22,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Electrophorus electricus acetylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50409820

(CHEMBL2114386)Show SMILES Cc1cccc2CO[P@](=O)(OC[C@H]3O[C@H](C=C3)n3cc(C)c(=O)[nH]c3=O)Oc12 |r,c:15| Show InChI InChI=1S/C18H19N2O7P/c1-11-4-3-5-13-9-24-28(23,27-16(11)13)25-10-14-6-7-15(26-14)20-8-12(2)17(21)19-18(20)22/h3-8,14-15H,9-10H2,1-2H3,(H,19,21,22)/t14-,15+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards beef erythrocytes acetylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50146850

(5-Methyl-1-[5-(3-oxo-1H-2,4-dioxa-3lambda*5*-phosp...)Show SMILES Cc1cn(C2OC(COP3(=O)OCc4ccc5ccccc5c4O3)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C21H19N2O7P/c1-13-10-23(21(25)22-20(13)24)18-9-8-16(29-18)12-28-31(26)27-11-15-7-6-14-4-2-3-5-17(14)19(15)30-31/h2-10,16,18H,11-12H2,1H3,(H,22,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Electrophorus electricus acetylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50146862

(1-[4-Azido-5-(8-methyl-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cccc2COP(=O)(OCC3OC(CC3N=[N+]=[N-])n3cc(C)c(=O)[nH]c3=O)Oc12 Show InChI InChI=1S/C18H20N5O7P/c1-10-4-3-5-12-8-27-31(26,30-16(10)12)28-9-14-13(21-22-19)6-15(29-14)23-7-11(2)17(24)20-18(23)25/h3-5,7,13-15H,6,8-9H2,1-2H3,(H,20,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards mouse butyrylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50146862

(1-[4-Azido-5-(8-methyl-2-oxo-4H-2lambda*5*-benzo[1...)Show SMILES Cc1cccc2COP(=O)(OCC3OC(CC3N=[N+]=[N-])n3cc(C)c(=O)[nH]c3=O)Oc12 Show InChI InChI=1S/C18H20N5O7P/c1-10-4-3-5-12-8-27-31(26,30-16(10)12)28-9-14-13(21-22-19)6-15(29-14)23-7-11(2)17(24)20-18(23)25/h3-5,7,13-15H,6,8-9H2,1-2H3,(H,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibitory activity towards calf serum acetylcholinesterase |
J Med Chem 47: 2839-52 (2004)
Article DOI: 10.1021/jm031032a
BindingDB Entry DOI: 10.7270/Q2P84CN5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data