

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424045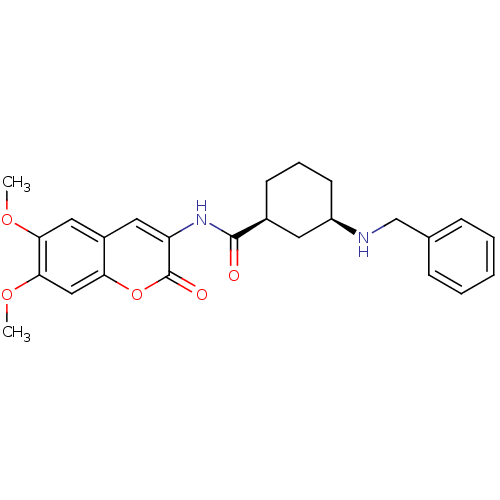 (CHEMBL2314726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed-type reversible inhibition of bovine acetylcholinesterase using S-acetylthiocholine as substrate incubated for 20 mins prior to substrate addit... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424054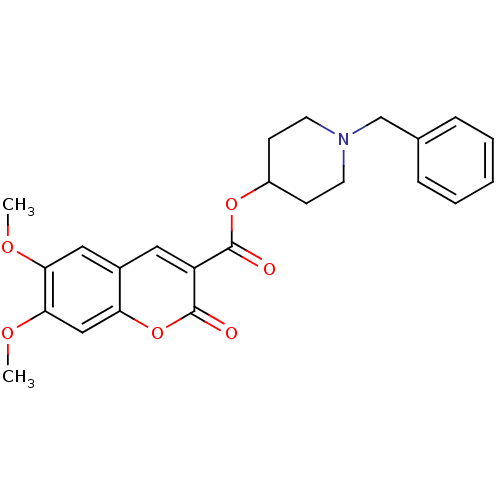 (CHEMBL2314720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424058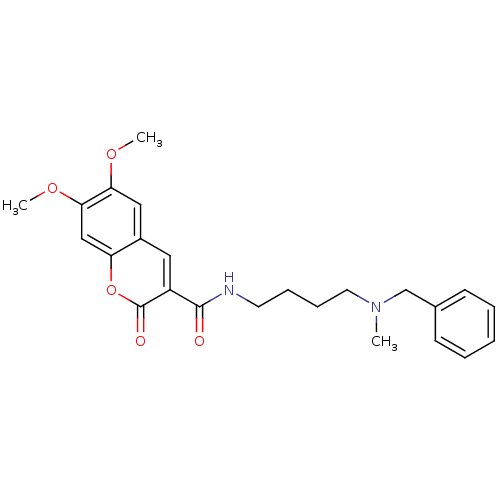 (CHEMBL2314429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424042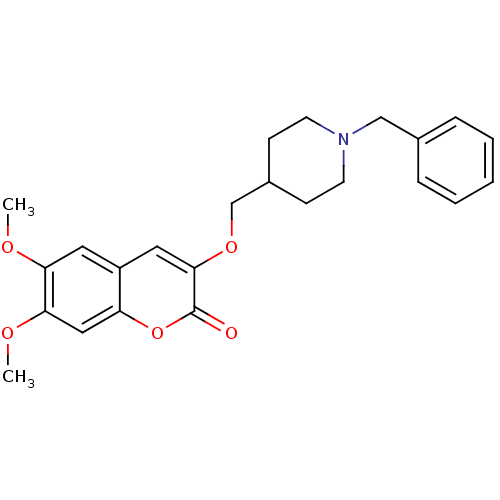 (CHEMBL2314731) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424055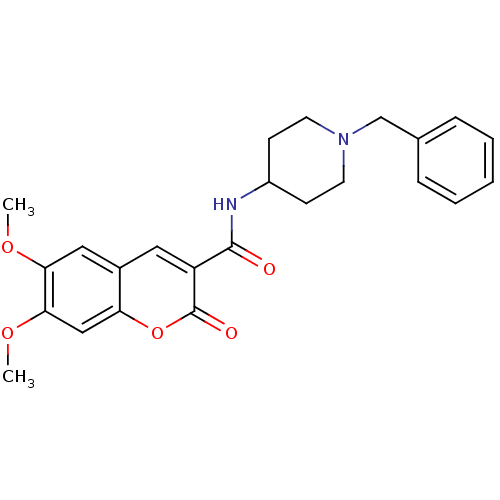 (CHEMBL2314432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424046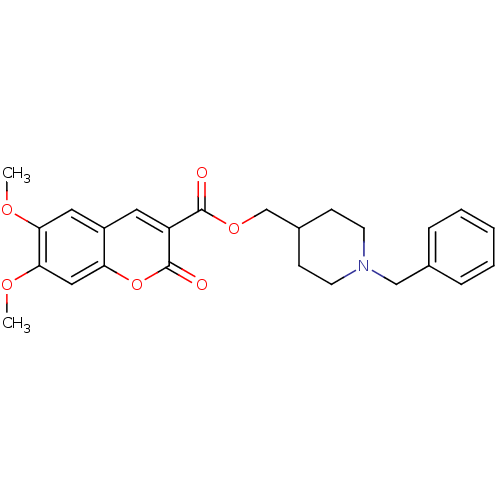 (CHEMBL2314721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424059 (CHEMBL2314428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424057 (CHEMBL2314430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10620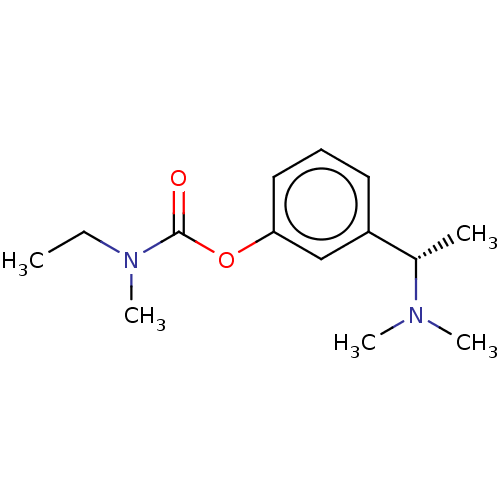 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424050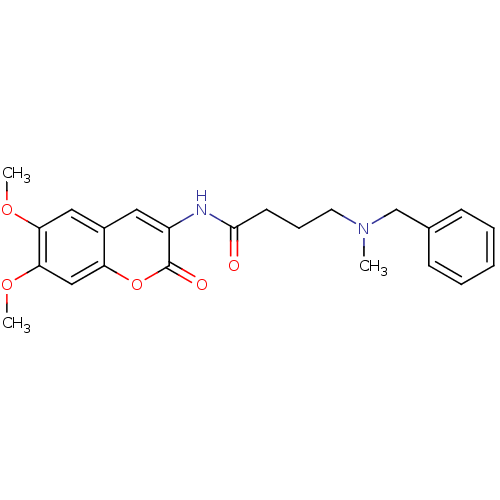 (CHEMBL2314725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424047 (CHEMBL2311571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50424042 (CHEMBL2314731) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50424044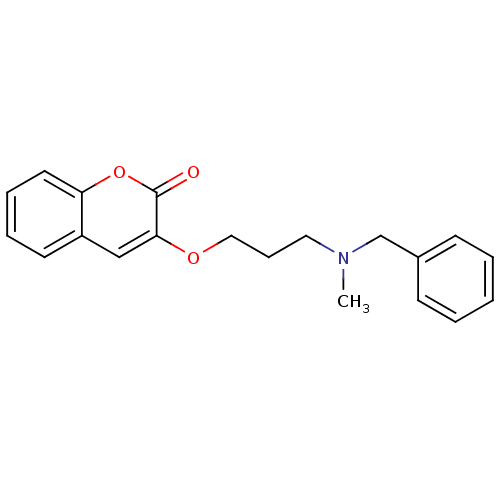 (CHEMBL2314727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424056 (CHEMBL2314431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424043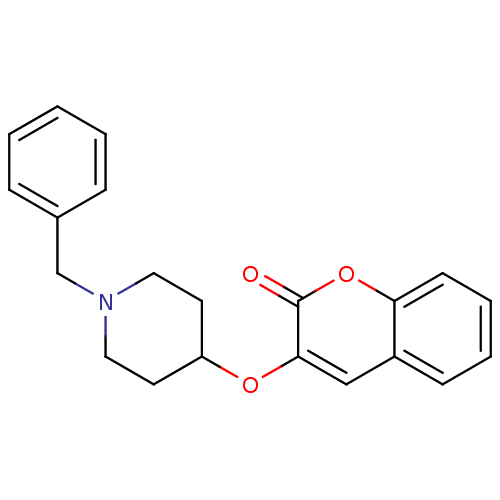 (CHEMBL2314728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424044 (CHEMBL2314727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50424046 (CHEMBL2314721) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424049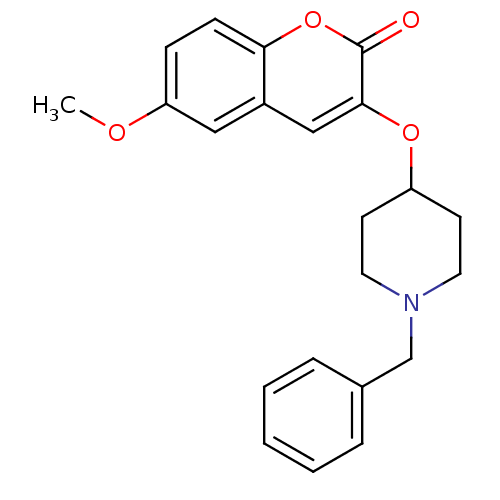 (CHEMBL2314729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50424043 (CHEMBL2314728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424060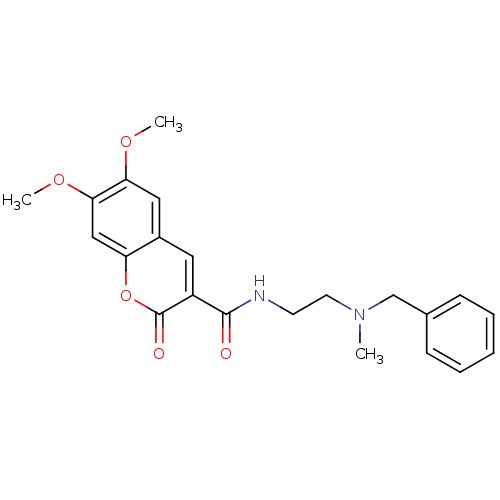 (CHEMBL2314427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424048 (CHEMBL2314730) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424051 (CHEMBL2314724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424053 (CHEMBL2314722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50424045 (CHEMBL2314726) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of equine butyrylcholinesterase using butyrylthicholine as substrate incubated for 20 mins prior to substrate addition measured after 3 mi... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424052 (CHEMBL2314723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||