 Found 86 hits Enz. Inhib. hit(s) with all data for entry = 50042476
Found 86 hits Enz. Inhib. hit(s) with all data for entry = 50042476 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50066789
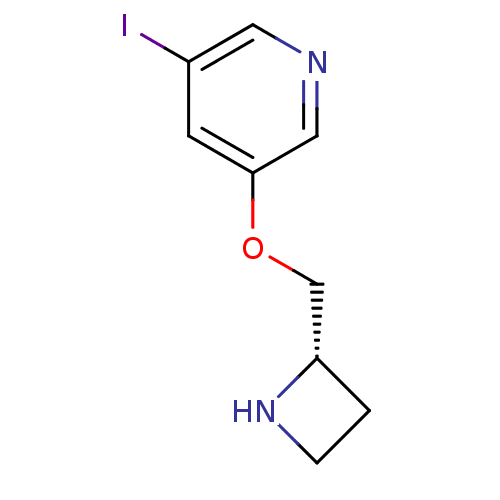
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain membrane |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR in rat cortex |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50067424
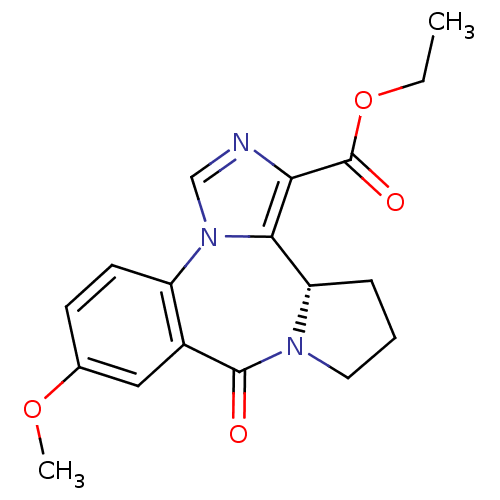
((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50142570
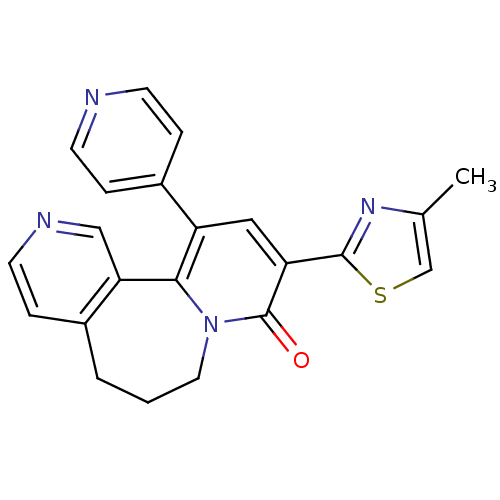
(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50179998
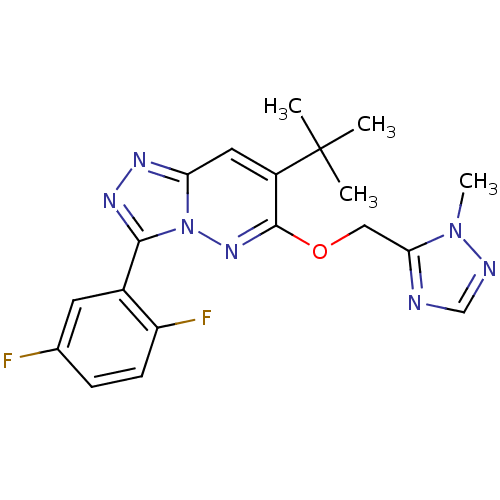
(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50067424

((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50426571
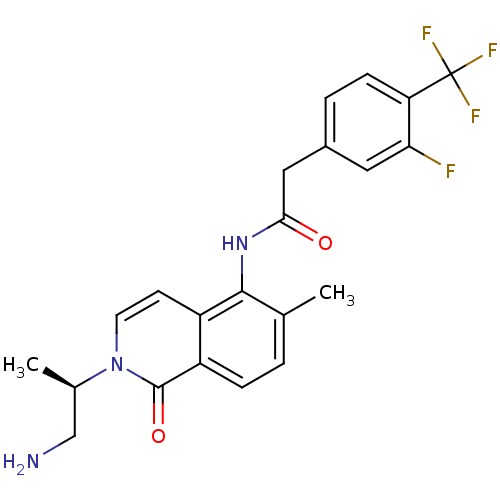
(CHEMBL2324343)Show SMILES C[C@H](CN)n1ccc2c(NC(=O)Cc3ccc(c(F)c3)C(F)(F)F)c(C)ccc2c1=O |r| Show InChI InChI=1S/C22H21F4N3O2/c1-12-3-5-16-15(7-8-29(21(16)31)13(2)11-27)20(12)28-19(30)10-14-4-6-17(18(23)9-14)22(24,25)26/h3-9,13H,10-11,27H2,1-2H3,(H,28,30)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of P2X7 receptor (unknown origin) assessed as inhibition of IL1beta production |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50426573
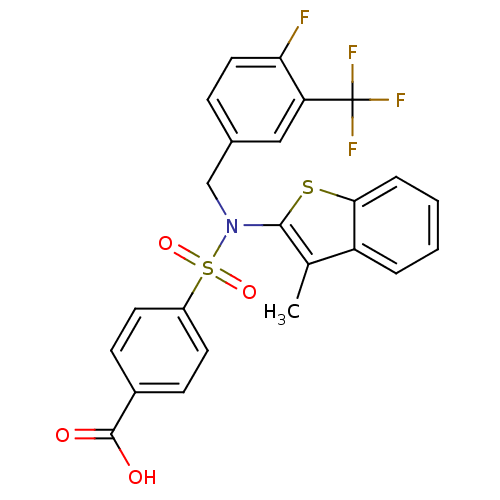
(CHEMBL2324349 | US9434711, 306)Show SMILES Cc1c(sc2ccccc12)N(Cc1ccc(F)c(c1)C(F)(F)F)S(=O)(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H17F4NO4S2/c1-14-18-4-2-3-5-21(18)34-22(14)29(13-15-6-11-20(25)19(12-15)24(26,27)28)35(32,33)17-9-7-16(8-10-17)23(30)31/h2-12H,13H2,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TRPM8 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50331290
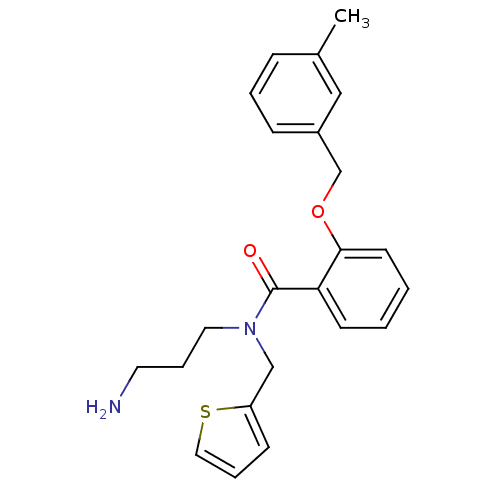
(CHEMBL1289953 | N-(3-aminopropyl)-2-(3-methylbenzy...)Show InChI InChI=1S/C23H26N2O2S/c1-18-7-4-8-19(15-18)17-27-22-11-3-2-10-21(22)23(26)25(13-6-12-24)16-20-9-5-14-28-20/h2-5,7-11,14-15H,6,12-13,16-17,24H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TRPM8 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Intermediate conductance calcium-activated potassium channel protein 4
(Homo sapiens (Human)) | BDBM50371391
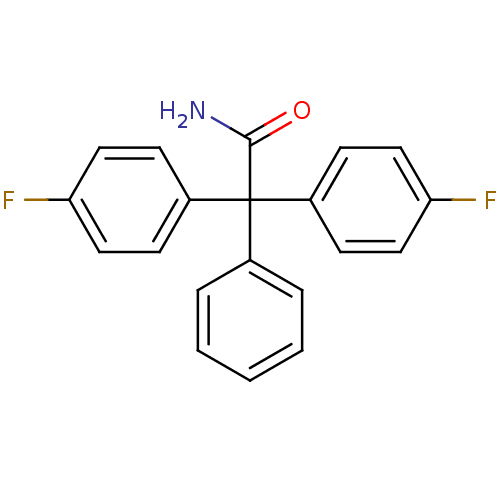
(SENICAPOC)Show InChI InChI=1S/C20H15F2NO/c21-17-10-6-15(7-11-17)20(19(23)24,14-4-2-1-3-5-14)16-8-12-18(22)13-9-16/h1-13H,(H2,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Kca 3.1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50000766

(CHEMBL12 | DIAZEPAM | US9271961, Diazepam)Show InChI InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant GABAA alpha5beta3gamma2 expressed in Xenopous laevis oocytes by patch clamp technique |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50426572
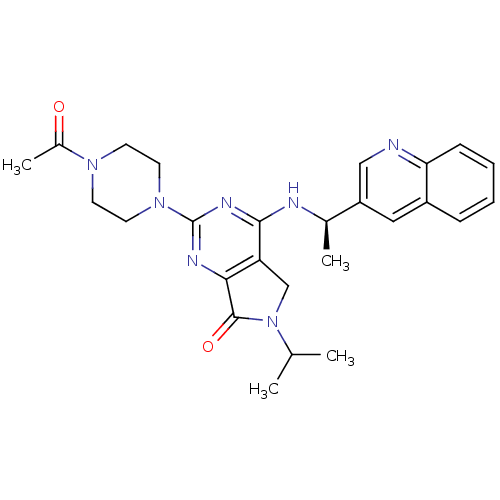
(CHEMBL2324342)Show SMILES CC(C)N1Cc2c(nc(nc2N[C@H](C)c2cnc3ccccc3c2)N2CCN(CC2)C(C)=O)C1=O |r| Show InChI InChI=1S/C26H31N7O2/c1-16(2)33-15-21-23(25(33)35)29-26(32-11-9-31(10-12-32)18(4)34)30-24(21)28-17(3)20-13-19-7-5-6-8-22(19)27-14-20/h5-8,13-14,16-17H,9-12,15H2,1-4H3,(H,28,29,30)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 expressed in rat liver endothelium cells assessed as inhibition of the intracellular calcium increase after 30 to 4... |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50379716
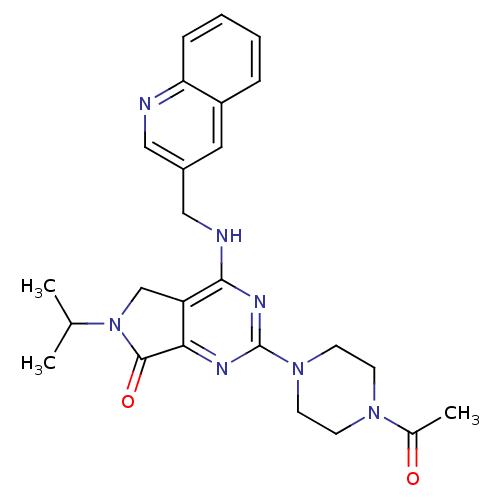
(CHEMBL2011125)Show SMILES CC(C)N1Cc2c(nc(nc2NCc2cnc3ccccc3c2)N2CCN(CC2)C(C)=O)C1=O Show InChI InChI=1S/C25H29N7O2/c1-16(2)32-15-20-22(24(32)34)28-25(31-10-8-30(9-11-31)17(3)33)29-23(20)27-14-18-12-19-6-4-5-7-21(19)26-13-18/h4-7,12-13,16H,8-11,14-15H2,1-3H3,(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 expressed in rat liver endothelium cells assessed as inhibition of the intracellular calcium increase after 30 to 4... |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50344821
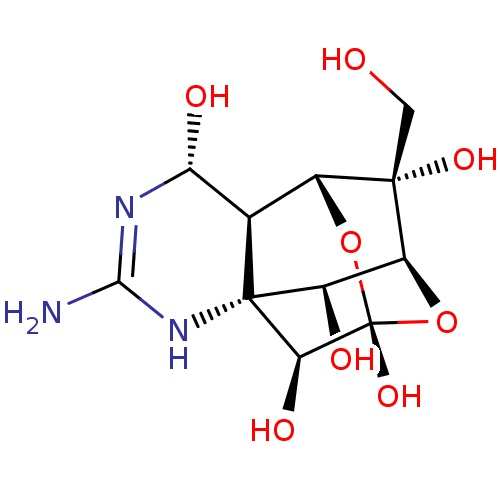
(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.7 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 2 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 3 subunit alpha
(Homo sapiens (Human)) | BDBM50426577
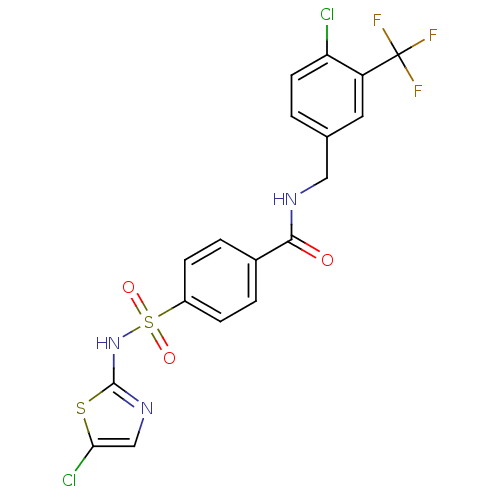
(CHEMBL2324354)Show SMILES FC(F)(F)c1cc(CNC(=O)c2ccc(cc2)S(=O)(=O)Nc2ncc(Cl)s2)ccc1Cl Show InChI InChI=1S/C18H12Cl2F3N3O3S2/c19-14-6-1-10(7-13(14)18(21,22)23)8-24-16(27)11-2-4-12(5-3-11)31(28,29)26-17-25-9-15(20)30-17/h1-7,9H,8H2,(H,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human voltage-gated Na channel 1.3 |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 4 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.4 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 11 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.9 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 3 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 8 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.6 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 10 subunit alpha
(Homo sapiens (Human)) | BDBM50330932
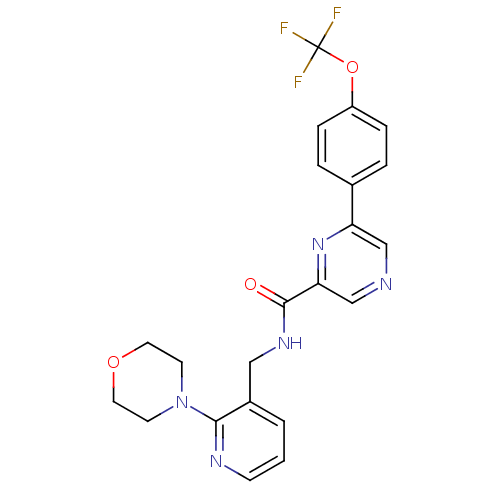
(CHEMBL1276882 | N-((2-Morpholin-4-yl-pyridin-3-yl)...)Show SMILES FC(F)(F)Oc1ccc(cc1)-c1cncc(n1)C(=O)NCc1cccnc1N1CCOCC1 Show InChI InChI=1S/C22H20F3N5O3/c23-22(24,25)33-17-5-3-15(4-6-17)18-13-26-14-19(29-18)21(31)28-12-16-2-1-7-27-20(16)30-8-10-32-11-9-30/h1-7,13-14H,8-12H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.8 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 10 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.8 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50344821

(10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...)Show SMILES NC1=N[C@H](O)[C@H]2[C@H]3O[C@]4(O)O[C@@H]([C@@H](O)[C@@]2(N1)[C@@H]4O)[C@]3(O)CO |r,t:1,TLB:13:12:16:6.7.5,18:11:16:6.7.5,18:6:16:11.10.12,19:18:7:16.14.5,20:18:7:16.14.5,THB:12:11:7:16.14.5,3:5:16:11.10.12| Show InChI InChI=1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium channel subfamily K member 9
(Homo sapiens (Human)) | BDBM50426570
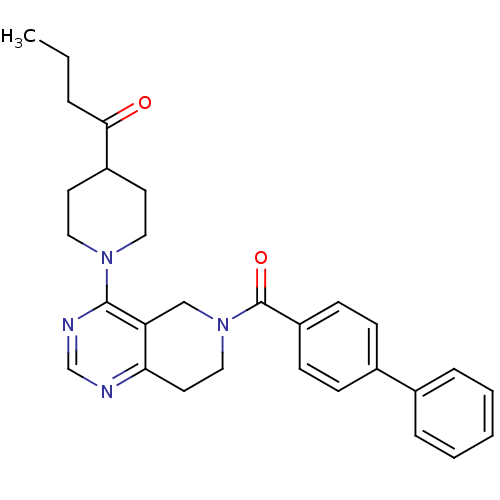
(CHEMBL2324344)Show SMILES CCCC(=O)C1CCN(CC1)c1ncnc2CCN(Cc12)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C29H32N4O2/c1-2-6-27(34)23-13-16-32(17-14-23)28-25-19-33(18-15-26(25)30-20-31-28)29(35)24-11-9-22(10-12-24)21-7-4-3-5-8-21/h3-5,7-12,20,23H,2,6,13-19H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TASK-3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50390072
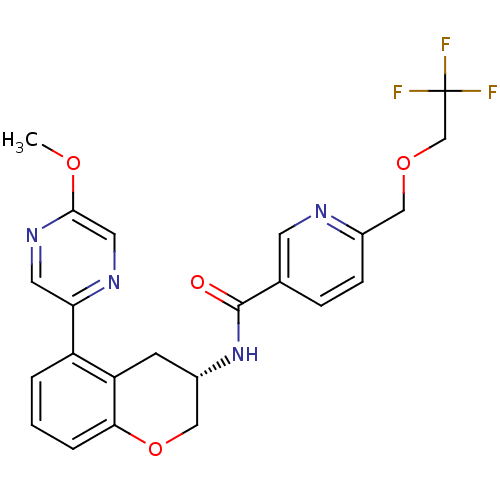
(CHEMBL2069427)Show SMILES COc1cnc(cn1)-c1cccc2OC[C@H](Cc12)NC(=O)c1ccc(COCC(F)(F)F)nc1 |r| Show InChI InChI=1S/C23H21F3N4O4/c1-32-21-10-28-19(9-29-21)17-3-2-4-20-18(17)7-16(12-34-20)30-22(31)14-5-6-15(27-8-14)11-33-13-23(24,25)26/h2-6,8-10,16H,7,11-13H2,1H3,(H,30,31)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human voltage-gated Na channel 1.7 by cell based patch clamp technique |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50426578
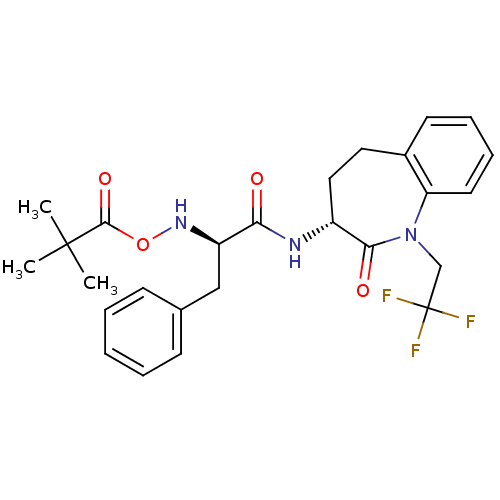
(CHEMBL2324352)Show SMILES CC(C)(C)C(=O)ON[C@H](Cc1ccccc1)C(=O)N[C@@H]1CCc2ccccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H30F3N3O4/c1-25(2,3)24(35)36-31-20(15-17-9-5-4-6-10-17)22(33)30-19-14-13-18-11-7-8-12-21(18)32(23(19)34)16-26(27,28)29/h4-12,19-20,31H,13-16H2,1-3H3,(H,30,33)/t19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human voltage-gated Na channel 1.7 expressed in HEK293 cells |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily B member 2
(Homo sapiens (Human)) | BDBM50004798
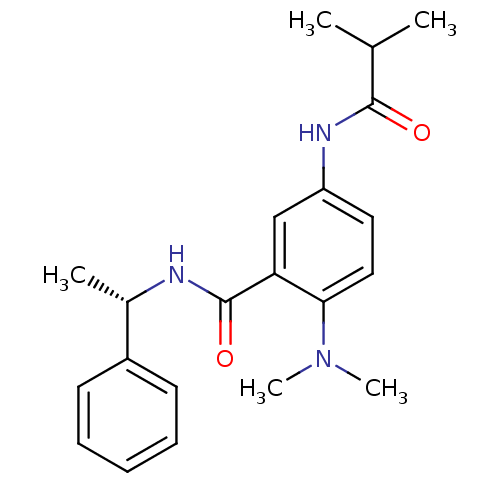
(CHEMBL2324356)Show SMILES CC(C)C(=O)Nc1ccc(N(C)C)c(c1)C(=O)N[C@@H](C)c1ccccc1 |r| Show InChI InChI=1S/C21H27N3O2/c1-14(2)20(25)23-17-11-12-19(24(4)5)18(13-17)21(26)22-15(3)16-9-7-6-8-10-16/h6-15H,1-5H3,(H,22,26)(H,23,25)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated K channel 2.2 (unknown origin) by automated patch clamp assay |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50300204
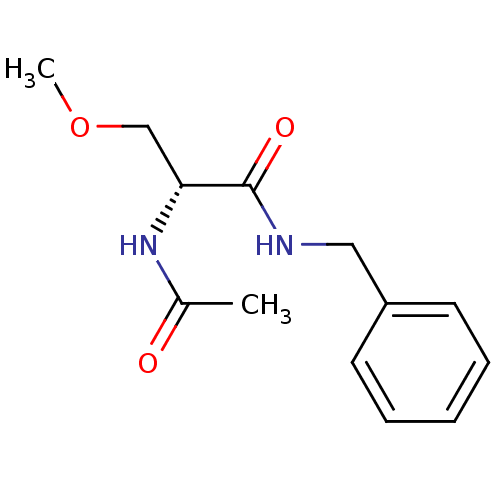
((2R)-2-acetylamino-N-benzyl-3-methoxypropanamide |...)Show InChI InChI=1S/C13H18N2O3/c1-10(16)15-12(9-18-2)13(17)14-8-11-6-4-3-5-7-11/h3-7,12H,8-9H2,1-2H3,(H,14,17)(H,15,16)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human inactivated voltage-gated Na channel 1.7 expressed in HEK293 cells by patch-clamp technique |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium channel subfamily K member 3
(Homo sapiens (Human)) | BDBM50426570

(CHEMBL2324344)Show SMILES CCCC(=O)C1CCN(CC1)c1ncnc2CCN(Cc12)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C29H32N4O2/c1-2-6-27(34)23-13-16-32(17-14-23)28-25-19-33(18-15-26(25)30-20-31-28)29(35)24-11-9-22(10-12-24)21-7-4-3-5-8-21/h3-5,7-12,20,23H,2,6,13-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TASK-1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium channel subfamily K member 2
(Homo sapiens (Human)) | BDBM79180
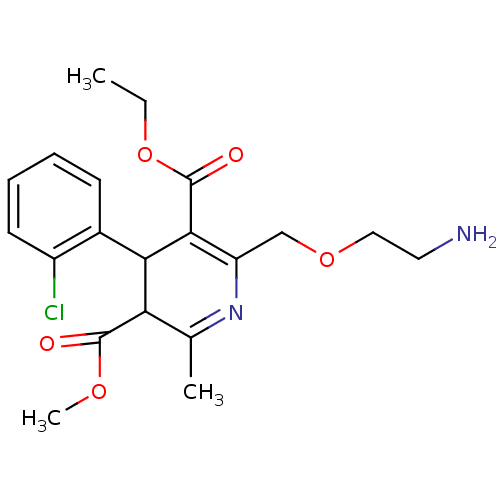
(2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methy...)Show SMILES CCOC(=O)C1=C(COCCN)N=C(C)C(C1c1ccccc1Cl)C(=O)OC |c:5,t:12| Show InChI InChI=1S/C20H25ClN2O5/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h5-8,16-17H,4,9-11,22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TREK-1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 3 subunit alpha
(Rattus norvegicus) | BDBM50300204

((2R)-2-acetylamino-N-benzyl-3-methoxypropanamide |...)Show InChI InChI=1S/C13H18N2O3/c1-10(16)15-12(9-18-2)13(17)14-8-11-6-4-3-5-7-11/h3-7,12H,8-9H2,1-2H3,(H,14,17)(H,15,16)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to rat inactivated voltage-gated Na channel 1.3 expressed in human HEK293 cells by patch-clamp technique |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM86751

(CHEMBL14935 | LY 293558 | LY-293558)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](CCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O2/c19-13(20)11-6-10-5-8(1-3-9(10)7-14-11)2-4-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8-,9+,10-,11+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GluK1 receptor (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 10 subunit alpha
(Homo sapiens (Human)) | BDBM50426578

(CHEMBL2324352)Show SMILES CC(C)(C)C(=O)ON[C@H](Cc1ccccc1)C(=O)N[C@@H]1CCc2ccccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H30F3N3O4/c1-25(2,3)24(35)36-31-20(15-17-9-5-4-6-10-17)22(33)30-19-14-13-18-11-7-8-12-21(18)32(23(19)34)16-26(27,28)29/h4-12,19-20,31H,13-16H2,1-3H3,(H,30,33)/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human voltage-gated Na channel 1.8 expressed in HEK293 cells |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily C member 1
(Rattus norvegicus) | BDBM50254790
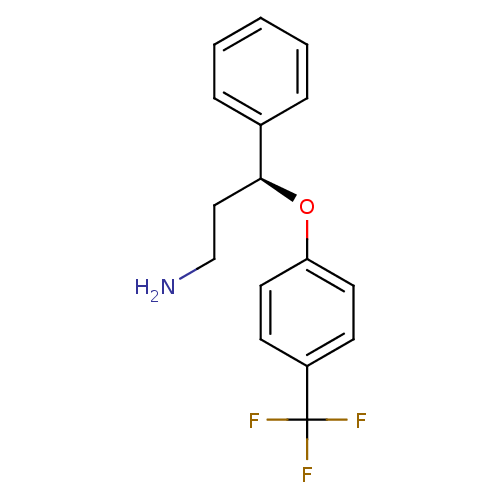
((S)-Norfluoxetine | CHEMBL465123 | NORFLUOXETINE)Show InChI InChI=1S/C16H16F3NO/c17-16(18,19)13-6-8-14(9-7-13)21-15(10-11-20)12-4-2-1-3-5-12/h1-9,15H,10-11,20H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat voltage-gated K channel 3.1 expressed in CHO cells by patch clamp assay |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50371391

(SENICAPOC)Show InChI InChI=1S/C20H15F2NO/c21-17-10-6-15(7-11-17)20(19(23)24,14-4-2-1-3-5-14)16-8-12-18(22)13-9-16/h1-13H,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50371391

(SENICAPOC)Show InChI InChI=1S/C20H15F2NO/c21-17-10-6-15(7-11-17)20(19(23)24,14-4-2-1-3-5-14)16-8-12-18(22)13-9-16/h1-13H,(H2,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated K channel 1.5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily KQT member 1
(Homo sapiens (Human)) | BDBM50371391

(SENICAPOC)Show InChI InChI=1S/C20H15F2NO/c21-17-10-6-15(7-11-17)20(19(23)24,14-4-2-1-3-5-14)16-8-12-18(22)13-9-16/h1-13H,(H2,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated K channel 7.1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50371391

(SENICAPOC)Show InChI InChI=1S/C20H15F2NO/c21-17-10-6-15(7-11-17)20(19(23)24,14-4-2-1-3-5-14)16-8-12-18(22)13-9-16/h1-13H,(H2,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.7 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 8 subunit alpha
(Homo sapiens (Human)) | BDBM50371391

(SENICAPOC)Show InChI InChI=1S/C20H15F2NO/c21-17-10-6-15(7-11-17)20(19(23)24,14-4-2-1-3-5-14)16-8-12-18(22)13-9-16/h1-13H,(H2,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of voltage-gated Na channel 1.6 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data